The Asia Pacific pharmacovigilance market size was exhibited at USD 2.85 billion in 2023 and is projected to hit around USD 6.56 billion by 2033, growing at a CAGR of 8.7% during the forecast period 2024 to 2033.
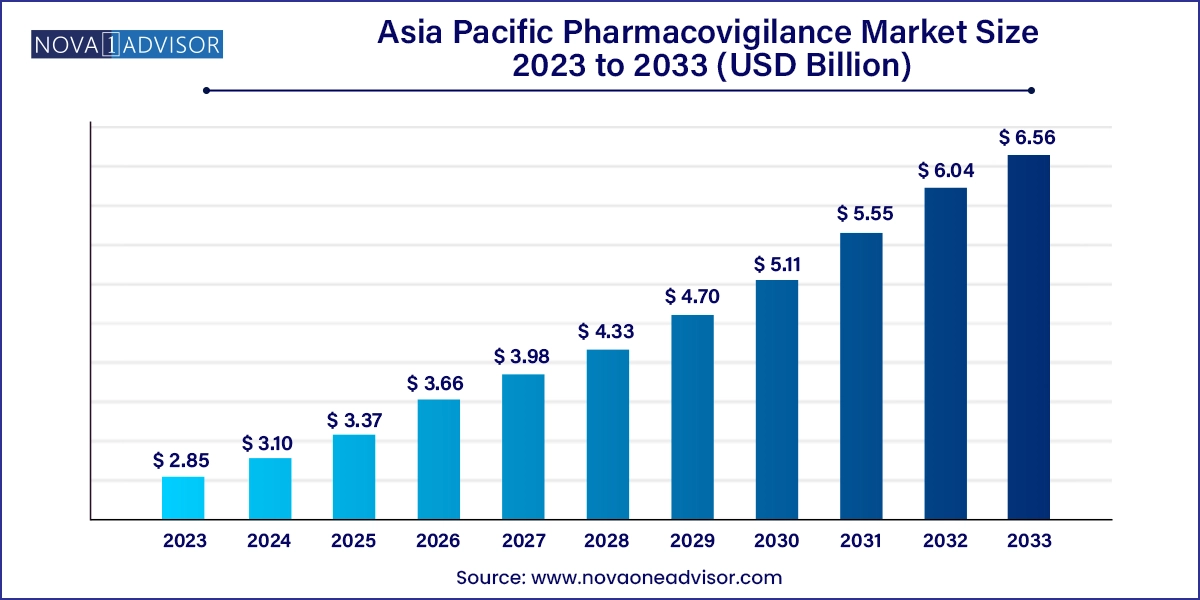
The Asia Pacific pharmacovigilance market is rapidly evolving, supported by growing pharmaceutical R&D, expanding clinical trial activity, and the region’s increasing contribution to global drug manufacturing and innovation. Pharmacovigilance—the science and activities related to detecting, evaluating, understanding, and preventing adverse drug reactions (ADRs)—has become a pivotal component in drug development and healthcare delivery across Asia Pacific.
Traditionally dominated by North America and Europe, the global pharmacovigilance landscape is witnessing a significant geographic shift, with countries like India, China, Japan, and South Korea emerging as vital contributors. Governments across the region are now enforcing stronger post-marketing surveillance requirements, while multinational pharmaceutical companies are investing in local safety infrastructure to meet global compliance standards.
The growing volume of clinical trials, particularly in oncology, neurology, and infectious diseases, is amplifying the demand for safety monitoring. With an expanding patient population and increasing access to prescription drugs, pharmacovigilance systems are required not just to ensure regulatory compliance but also to protect public health. This has opened new growth avenues for contract outsourcing firms, AI-based pharmacovigilance platforms, and data-driven safety analytics providers.
Importantly, pharmacovigilance is transitioning from a cost center to a strategic differentiator. Companies leveraging advanced safety analytics, real-world evidence (RWE), and electronic health records (EHRs) for signal detection are better positioned to respond proactively to safety issues, thus enhancing product longevity and brand trust. The Asia Pacific market is poised for robust growth through this decade, underpinned by its rising healthcare digitization and harmonization with international regulatory standards.
Globalization of Drug Safety Compliance: Increasing alignment of regional regulatory frameworks with ICH and WHO guidelines for pharmacovigilance reporting and monitoring.
Expansion of Clinical Trials: Surge in Phase III and IV trials across India, China, and South Korea driving demand for sophisticated pharmacovigilance services.
AI-Enabled Signal Detection: Adoption of AI and machine learning algorithms for automation of ADR reporting and predictive analytics.
Rise of Local PV Outsourcing Firms: Emergence of domestic CROs and pharmacovigilance service providers offering scalable, cost-effective safety operations.
Real-World Data Integration: Increasing use of hospital data, EHRs, and patient registries for post-marketing surveillance and adverse event tracking.
Digital Adverse Event Reporting Tools: Deployment of mobile apps and web-based portals for direct patient ADR reporting.
COVID-19 and Vaccine Safety Monitoring: Strengthened infrastructure and vigilance systems due to pandemic-driven pharmacovigilance efforts.
| Report Coverage | Details |
| Market Size in 2024 | USD 3.10 Billion |
| Market Size by 2033 | USD 6.56 Billion |
| Growth Rate From 2024 to 2033 | CAGR of 8.7% |
| Base Year | 2023 |
| Forecast Period | 2024-2033 |
| Segments Covered | Product life cycle, Service Provider, Type, End use, Therapeutic Area, Process Flow, Country |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Country scope | Japan; China; India; Australia; South Korea; Thailand |
| Key Companies Profiled | Dr. Reddy’s Laboratories Ltd.; Sun Pharmaceutical Industries Ltd.; Cipla Inc.; Aurobindo Pharma.; Asymchem Laboratories; Reyoung Pharmaceutical; CSPC Pharmaceutical Group Limited; Otsuka Pharmaceutical Australia Pty Ltd.; GC Biopharma Corp.; Chong Kun Dang Pharmaceutical Corporation. |
Boom in Clinical Trials Across Asia Pacific
One of the most significant drivers in the Asia Pacific pharmacovigilance market is the exponential rise in clinical trial activity across the region. Countries like India, China, and South Korea have become hotbeds for global clinical trials due to their large patient pools, cost-effective research environments, and improving regulatory transparency. As these trials progress through later phases, pharmacovigilance becomes critical to monitor trial-related adverse events, ensure patient safety, and secure regulatory approvals.
For example, India’s New Drugs and Clinical Trials Rules, 2019 mandate rigorous safety reporting mechanisms, while China’s National Medical Products Administration (NMPA) now emphasizes pharmacovigilance compliance for both domestic and foreign manufacturers. These developments significantly increase the need for advanced pharmacovigilance platforms, staff training, and AI-integrated solutions to manage high volumes of safety data efficiently.
Fragmented Regulatory Framework Across Countries
Despite considerable progress, the pharmacovigilance ecosystem in Asia Pacific is still hindered by non-uniform and evolving regulatory landscapes. While countries like Japan and Australia have mature pharmacovigilance systems aligned with ICH guidelines, other markets like Thailand and Indonesia are still developing their frameworks. This lack of standardization increases complexity for pharmaceutical companies conducting multi-country trials or post-marketing surveillance across the region.
Inconsistencies in ADR reporting requirements, data formats, timelines, and language barriers add to the operational burden. Additionally, limited awareness among healthcare professionals and the general public regarding adverse event reporting reduces the volume and quality of data collected. These challenges necessitate customized pharmacovigilance strategies for each market, driving up costs and limiting scalability.
AI-Driven Pharmacovigilance Platforms for Scalable Growth
As the volume of adverse event data grows, the application of AI and natural language processing (NLP) in pharmacovigilance presents a transformative opportunity for the Asia Pacific market. AI-based platforms can automate case intake, coding, causality assessment, and signal detection reducing manual workload and minimizing errors.
In India and China, startups and global tech companies are piloting platforms that integrate with EHR systems and tap into unstructured data from call centers, social media, and medical literature. These tools offer predictive safety insights, enhance regulatory readiness, and accelerate decision-making. With supportive policies around digital health, increasing cloud infrastructure adoption, and growing acceptance of AI by regulators, AI-enabled pharmacovigilance is set to disrupt traditional workflows and offer significant competitive advantage.
Phase IV pharmacovigilance held the dominant share in the Asia Pacific market due to the region’s increasing focus on post-marketing drug surveillance and patient safety. Once drugs are approved and released to the market, Phase IV activities ensure the long-term safety and effectiveness of these products in real-world populations. Regulatory bodies in countries like Japan and South Korea have implemented strict pharmacovigilance obligations post-approval, requiring manufacturers to conduct post-marketing studies, submit periodic safety update reports (PSURs), and manage risk mitigation plans. These requirements have led to robust growth in Phase IV pharmacovigilance services, particularly among multinationals launching products in the region.
Meanwhile, Phase III is experiencing the fastest growth, driven by the rising number of late-stage clinical trials across Asia Pacific. Countries like China and India are home to hundreds of Phase III studies annually, requiring constant safety monitoring. Regulatory agencies are increasing their oversight of adverse event data generated during clinical research. This growth is further accelerated by the influx of foreign pharmaceutical sponsors outsourcing safety monitoring to local CROs with Phase III expertise. Technologies like electronic data capture (EDC) and real-time safety signal monitoring are increasingly being deployed to enhance trial efficiency and compliance.
Contract outsourcing was the leading service provider category due to the rising trend of pharmaceutical companies outsourcing pharmacovigilance functions to local and regional CROs. Outsourcing enables firms to scale operations, reduce costs, and access specialized safety expertise without building internal teams. In Asia Pacific, a growing number of BPO and CRO firms offer end-to-end pharmacovigilance services, from case data management to signal detection and risk mitigation. The maturity of India’s outsourcing industry, the rise of Chinese pharmacovigilance consultancies, and Japan’s hybrid outsourcing models all support this segment’s dominance.
Nonetheless, in-house pharmacovigilance services are expected to grow, particularly among large pharmaceutical and biotech firms in Japan and China who seek tighter control over data privacy, regulatory reporting, and product lifecycle management. These organizations are investing in proprietary safety platforms, EHR integration, and AI-based signal detection to build internal pharmacovigilance capabilities. In-house systems also support real-time cross-functional collaboration, especially for complex or innovative therapies, including biologics and cell-based therapies.
Spontaneous reporting was the most utilized pharmacovigilance type in Asia Pacific, especially as the foundation for national drug safety surveillance systems. Countries like India (PvPI), China (CADRMP), and Japan (PMDA) have established centralized reporting portals and programs encouraging healthcare professionals and consumers to report suspected adverse reactions. These spontaneous reports are critical for early signal detection, regulatory review, and public health alerts.
However, EHR mining is growing rapidly as healthcare digitization expands across Asia Pacific. Hospitals and health systems are increasingly equipped with electronic medical record systems, especially in urban centers. Real-world data extracted from these records is proving valuable in detecting adverse events that may be underreported in traditional systems. For example, China’s health tech initiatives under the “Healthy China 2030” framework are enabling pilot projects using EHR mining for oncology and cardiovascular drugs. South Korea’s big data initiatives also aim to integrate EHRs into national pharmacovigilance networks.
Pharmaceutical companies were the largest end users of pharmacovigilance services, driven by their need to comply with global regulations and maintain post-marketing surveillance. These companies—especially multinationals—run large drug portfolios across multiple Asia Pacific countries, necessitating strong pharmacovigilance infrastructure. In addition to outsourcing safety operations, many pharma firms maintain regional safety hubs in countries like Singapore and India to manage local reporting and regulatory compliance.
Meanwhile, biotechnology companies are projected to grow fastest in the pharmacovigilance space. The biotech sector in Asia Pacific is thriving, with innovative therapies entering clinical trials in immunology, rare diseases, and oncology. As these firms move from early-stage development to late-phase trials and commercialization, they are under pressure to implement robust pharmacovigilance systems. Increasing regulatory focus on biologicals and personalized medicine products is further accelerating this trend.
Oncology continued to dominate the therapeutic segmentation due to the high incidence of cancer in Asia Pacific and the complexity of oncology drug regimens. Immuno-oncology, targeted therapies, and biosimilars demand rigorous safety monitoring due to their severe and often unpredictable adverse events. Regulatory authorities in Japan and Australia have outlined pharmacovigilance frameworks tailored for cancer drugs, including risk evaluation strategies and mandatory post-marketing safety studies.
Neurology is emerging as a high-growth therapeutic area for pharmacovigilance. With rising prevalence of neurological disorders such as Alzheimer’s, epilepsy, and Parkinson’s disease, more drugs are being developed and tested in this domain. These treatments often require chronic administration and involve unique safety considerations like psychiatric side effects or neurological deterioration. Asia Pacific’s aging population and improved diagnostic capabilities are contributing to this trend, prompting regulators and drug developers to expand safety monitoring efforts in neurology.
Signal detection, on the other hand, is the fastest-growing process area. With growing volumes of safety data from clinical trials, spontaneous reports, and EHRs, organizations are investing in AI-based systems that can identify emerging safety trends before they become widespread issues. Countries like India and China are encouraging proactive signal detection through public-private collaborations and digital health projects. For example, the Japan PMDA's investment in data mining tools for periodic signal analysis underscores the region’s emphasis on early risk identification.
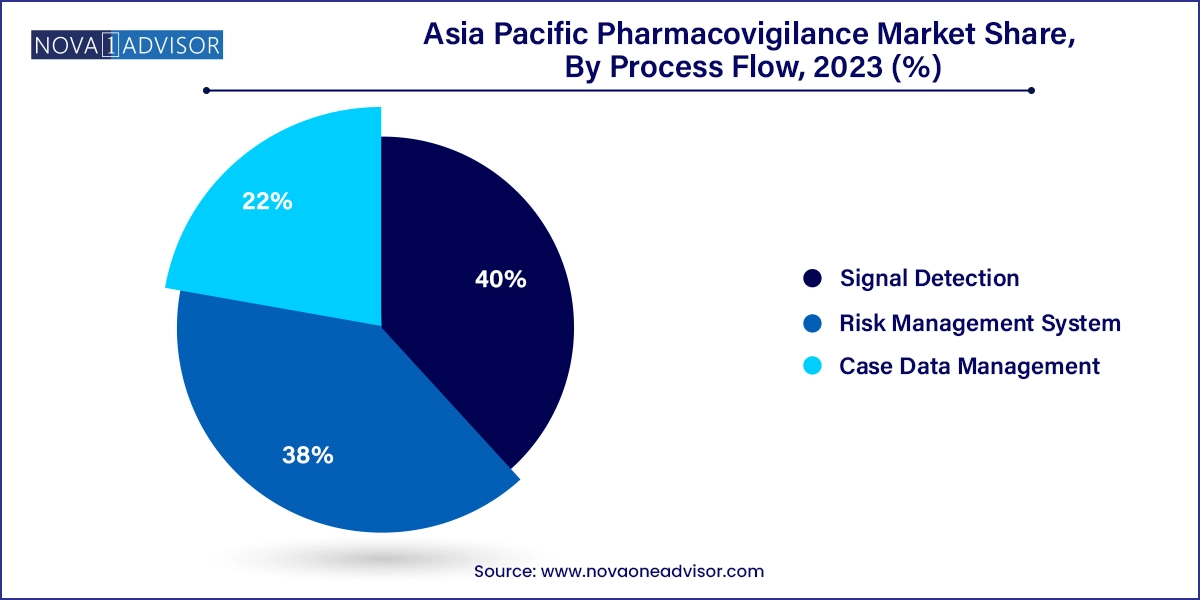
Case data management dominated process flow activities, reflecting its foundational role in pharmacovigilance operations. Collecting, validating, coding, and submitting individual case safety reports (ICSRs) are regulatory requirements across all major Asia Pacific countries. Most companies deploy standardized tools or third-party platforms to manage high volumes of cases across multiple languages and jurisdictions. The increasing use of automation in this area—such as auto-narrative generation and MedDRA coding—is improving turnaround time and efficiency.
Japan has one of the most advanced pharmacovigilance infrastructures in Asia. The Pharmaceuticals and Medical Devices Agency (PMDA) mandates detailed risk management plans and supports spontaneous reporting systems. Japanese pharma companies also invest heavily in in-house pharmacovigilance and predictive analytics.
China’s NMPA has dramatically reformed its drug safety regulations in recent years. The establishment of the CADRMP and integration with hospital data systems are positioning China as a growing pharmacovigilance hub. Domestic CROs are increasingly partnering with multinationals for local safety monitoring.
India’s Pharmacovigilance Programme of India (PvPI) is one of the most active national reporting systems globally. With a large outsourcing industry, India is a preferred destination for pharmacovigilance BPO services. Startups and software companies are also entering the space with AI-enabled solutions.
Australia's Therapeutic Goods Administration (TGA) follows strict pharmacovigilance standards aligned with ICH guidelines. The country has a strong clinical research infrastructure and uses national registries for post-marketing surveillance.
South Korea is a leader in digital health and big data integration. The Korea Institute of Drug Safety and Risk Management (KIDS) oversees ADR monitoring, and the government promotes EHR integration and real-world data analysis for signal detection.
Thailand is developing its pharmacovigilance capabilities with assistance from WHO and international agencies. The Thai FDA is working to improve reporting practices and harmonize its systems with ASEAN and ICH standards.
March 2025 – Novartis partnered with an Indian BPO to establish a pharmacovigilance center for Asia Pacific operations, focusing on automation and EHR integration.
January 2025 – Tata Consultancy Services announced the expansion of its AI-based pharmacovigilance suite in Japan and Australia to enhance signal detection.
November 2024 – China's NMPA launched a real-world data initiative for pharmacovigilance, linking hospital data with national safety reporting systems.
October 2024 – Samsung Biologics in South Korea initiated a collaboration with a local CRO to develop a pharmacovigilance infrastructure for its biosimilar products.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the Asia Pacific pharmacovigilance market.
Product Life Cycle
Service Provider
Type
End Use
Therapeutic Area
Process Flow
Country