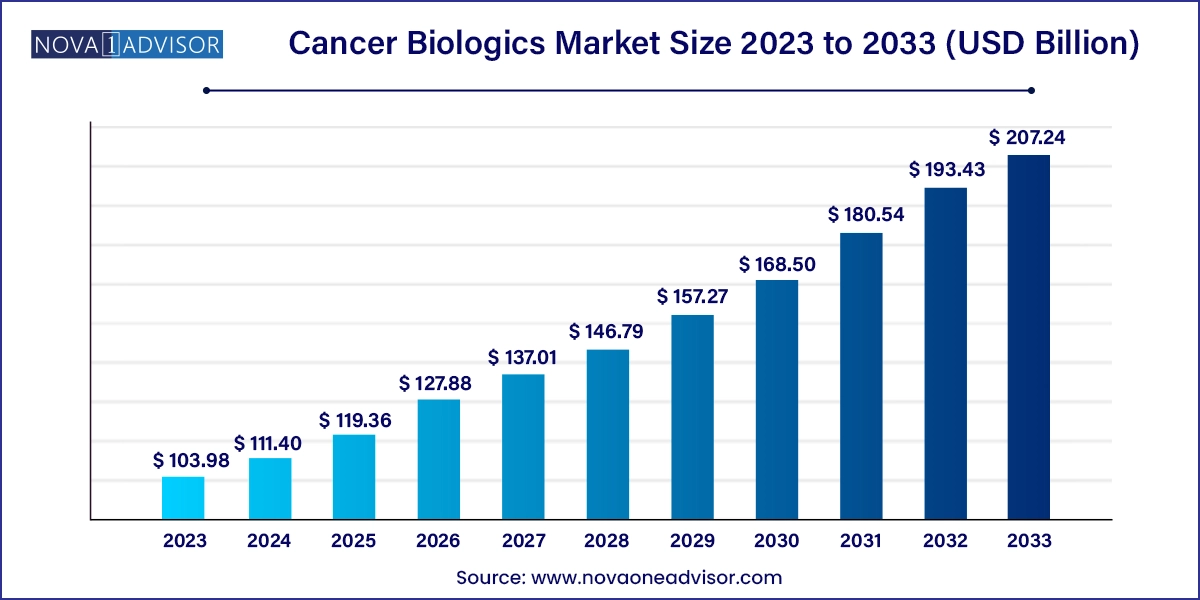
The global cancer biologics market size was valued at USD 103.98 billion in 2023 and is projected to surpass around USD 207.24 billion by 2033, registering a CAGR of 7.14% over the forecast period of 2024 to 2033.
The cancer biologics market encompasses a diverse array of therapeutic agents derived from biological sources, such as living organisms, cells, proteins, and antibodies, designed to target specific mechanisms involved in cancer growth and progression. Unlike traditional chemotherapy, biologics are tailored to interact with particular molecules within cancer cells or the surrounding environment, offering potentially higher efficacy and fewer side effects. Key biologics include monoclonal antibodies, cytokines, cancer vaccines, and targeted therapies that inhibit pathways critical to tumor survival. The market's growth is propelled by increasing incidences of cancer worldwide, technological advancements in biotechnology, and a shift towards personalized medicine. Regulatory support and robust research pipelines further fuel innovation, driving competition among pharmaceutical companies to develop novel biologics with improved therapeutic outcomes. As a result, the cancer biologics market continues to expand, offering new hope and treatment options for cancer patients globally.
Increasing Cancer Incidence: The rising prevalence of cancer worldwide is a significant driver for the demand for cancer biologics. As cancer rates continue to increase globally, there is a growing need for effective and targeted treatments.
Advancements in Biotechnology: Advances in biotechnology have led to the development of more sophisticated and effective biologic therapies for cancer. These include monoclonal antibodies, cytokines, vaccines, and other biologic agents that specifically target cancer cells or enhance the body's immune response against cancer.
Personalized Medicine: The shift towards personalized medicine has been facilitated by biologics. These treatments can be tailored to the genetic and molecular characteristics of individual patients, leading to better outcomes and reduced side effects compared to traditional chemotherapy.
Rising Investments in Research and Development: Pharmaceutical companies and research institutions are investing heavily in the development of new cancer biologics. This ongoing research has resulted in the discovery of novel targets and the development of innovative biologic therapies.
Government Initiatives and Support: Governments in many countries are supporting research and development in biologics through grants, funding, and regulatory incentives. This support accelerates the development and approval of new cancer biologics.
Increasing Healthcare Expenditure: As global healthcare expenditure rises, particularly in emerging economies, there is greater affordability and access to advanced biologic therapies for cancer treatment.
Efficacy and Safety Profile: Biologics often have a more favorable efficacy and safety profile compared to traditional chemotherapy. This has led to increased adoption by healthcare providers and patients seeking better treatment options.
Patent Expirations of Chemical Drugs: With patents expiring on some chemical drugs used for cancer treatment, there is a shift towards biologics which offer opportunities for pharmaceutical companies to enter and expand in the market.
| Report Attribute | Details |
| Market Size in 2024 | USD 111.40 Billion |
| Market Size by 2033 | USD 207.24 Billion |
| Growth Rate From 2024 to 2033 | CAGR of 7.14% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Drug Class, By Applications, and By End-use |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Key Companies Profiled | Abbott, Angel, Amgen, Inc, AstraZeneca, BioNTech, Bristol-Mayer Squibb Company, Dr. Reddy's Laboratories, Duality Biologics, Eli Lilly and Company, F.Hoffmann-La Roche Ltd., Gilead Sciences, Inc., Glenmark Pharmaceuticals Ltd, GSK plc., Ichnos Sciences Inc, Johnson & Johnson Services, Inc, Pfizer, Inc, TFC Therapeutics, and Others. |
Driver
Rising incidence of cancer
The increasing incidence of cancer can be the driving factor in the cancer biologics market. As the number of cancer patients rises in the cancer biologics market, pharmaceutical companies may invest more resources in research, developing, and commercializing biologics to meet the growing demand for cancer biologics.
Restraint
Risks associated with biologics
The risk associated with biologics can be a restraint to the cancer biologics market. People are more concerned about the health and the side effects of biologics, including allergy reactions and common infections. People who take biologics are more likely to get infections such as pneumonia, upper respiratory infections, urinary tract infections, skin infections, opportunistic infections, and types of infections that are less common in healthy people and more common in people whose immune systems are impaired. Some examples of such opportunistic infections include tuberculosis, hepatitis B, and fungal infections such as histoplasmosis.
Opportunity
Advancement in biotechnology
The advancement in biotechnology of cancer biologics can be an opportunity for the cancer biologics market. The advances in biotechnology include next-generation sequencing, also known as NGS, CRISPER-Cas9 gene editing, and single-cell analysis, which have enabled research to understand the genetic mutation driving cancer and develop biologics for cancer patients.
The monoclonal antibodies segment dominated the cancer biologics market in 2023. The monoclonal antibodies are segmented into conjugated monoclonal antibodies, naked monoclonal antibodies and bispecific monoclonal antibodies. Monoclonal antibodies are a category of proteinious structures made in the laboratory that can bind to certain targeted antigens on the surface of cancer cells. A monoclonal antibody is produced from a cell lineage made by cloning a unique white blood cell. Examples of monoclonal antibodies include trastuzumab (Herceptin) for HER2, which is useful for breast cancer, and rituximab for lymphoma cancer.
The cancer growth inhibitors segment is expected to grow to the highest CAGR in the cancer biologics market by application during the forecast period. The cancer growth inhibitors are segmented into tyrosine kinase inhibitors, mTOR inhibitors, and proteasome inhibitors. Cancer growth inhibitors are drugs that interfere with the growth and proliferation of cancer cells. They work by targeting specific molecules that are crucial for cancer sale survival and replication. These cancer inhibitors can act through various mechanisms, including blocking cell signaling pathways, inhibiting DNA replication, or promoting sale death in cancer cells.
The blood cancer segment dominated the cancer biologics market by application in 2023. Blood cancer is also known as hematological malignancy, which refers to cancers that affect the blood, bone marrow, and lymphatic system. There are different types of blood cancer, including leukemia, lymphoma, and multiple myeloma, each with its own sub-types and characteristics. This cancer typically involves the uncontrolled growth of abnormal cells in the areas disrupting the normal functioning of the blood and immune system; when the growth of cells is completed, then the gland is known as a cancer cell.
The lung cancer segment is projected to grow to the highest CAGR in the cancer biologics market by application during the forecast period. Lung cancer is a cancer that affects the lungs. It is the most common type of cancer, and this cancer is caused by smoking or exposure to secondhand smoke, air pollution, or certain chemicals. There are two types of lung cancer, non-small-cell lung cancer and small-cell lung cancer. Both cancers have different characteristics and treatments.
The hospitals segment dominated the cancer biologics market by end-user, in 2023. Hospitals always offer oncology department services, which include treatment and ensuring the availability of biologics. Many hospitals are engaged in cancer research and clinical trials for adopting new biologics. Additionally, the hospital provides cancer care services, including surgery, chemotherapy, diagnostics, and biological therapies. Also, the reimbursement policy is often in favor of hospitals providing cancer care services and incentivizing healthcare providers to offer cancer biologics in healthcare settings.
The cancer centers segment is expected to grow to the highest CAGR in the cancer biologics market by end-users during the forecast period. Cancer center offers multidisciplinary care, which involves collaboration among oncologists, researchers, surgeons, and other specialists according to the type of cancer, which provides personalized treatment plans for patients.
North America led the cancer biologics market in 2023. Geographic variations in cancer rates are assumed to be mostly due to variances in lifestyle factors, such as smoking, which are found to be connected with a considerable fraction of diagnoses in North America. The need for cancer biologics is increased by the rising incidence and prevalence of cancer among North American populations, which has led to a growth in the market for cancer biologics in the region.
Throughout the forecast period, the cancer biologics market by geography is expected to rise gradually in Asia Pacific. The demand for cancer biologics has surged due to the increasing cancer prevalence in the Asia Pacific population, and as a result, the market for cancer biologics is expanding in this region. Owing to the surge in demand for cancer biologics, the major participants in the Asia-Pacific cancer biologics market are Novartis International AG, Pfizer Inc., Roche Holding AG, Merck & Co., Inc., and AstraZeneca plc.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the Cancer Biologics market.
By Drug Class
By Applications
By End use
By Region