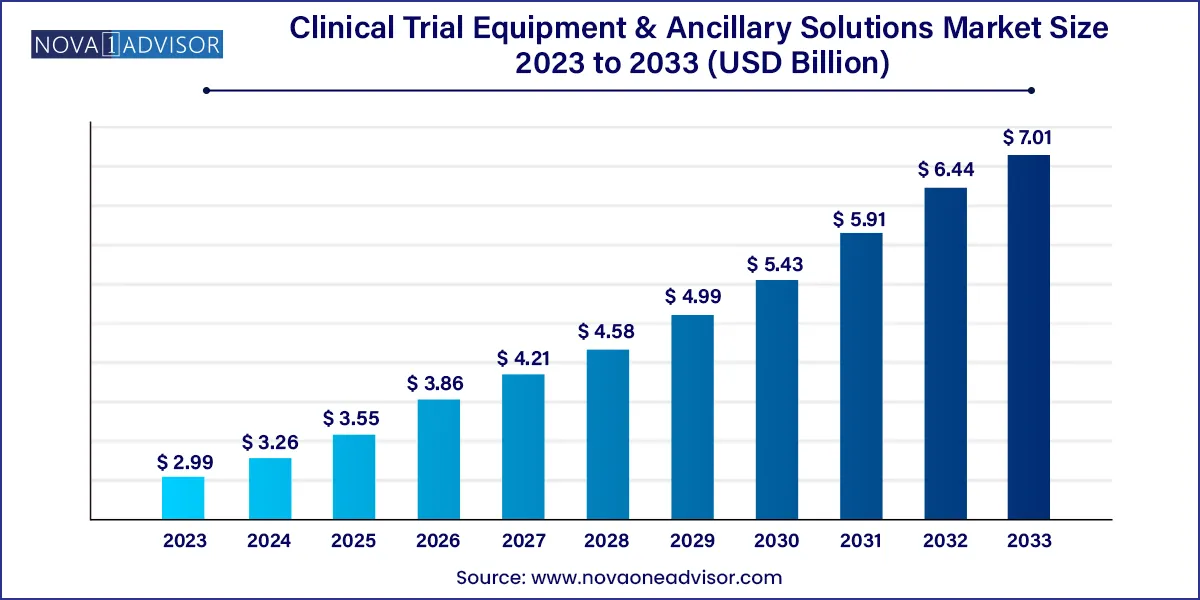
The global clinical trial equipment & ancillary solutions market size was exhibited at USD 2.99 billion in 2023 and is projected to hit around USD 7.01 billion by 2033, growing at a CAGR of 8.9% during the forecast period 2024 to 2033.
| Report Coverage | Details |
| Market Size in 2024 | USD 3.26 Billion |
| Market Size by 2033 | USD 7.01 Billion |
| Growth Rate From 2024 to 2033 | CAGR of 8.9% |
| Base Year | 2023 |
| Forecast Period | 2024-2033 |
| Segments Covered | Product, Phase, Region |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Regional Scope | North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa |
| Key Companies Profiled | Ancillare, LP; Imperial CRS, Inc.; Woodley Equipment Company Ltd.; Thermo Fisher Scientific, Inc.; Parexel International (MA) Corporation; Emsere (MediCapital Rent); Quipment SAS; IRM; Marken; Myonex; Yourway |
The global market has witnessed critical growth due to increasing R&D investments, the growing need for clinical trials, and the rapid growth of pharmaceutical and medical industries.
The COVID-19 outbreak temporarily hindered the process of clinical trials in 2020 due to lockdowns enforced by governments, which impacted the market negatively. However, rising demand for effective COVID-19 treatments and other disorders led to a growth in the number of trials. Companies and government authorities are launching clinical trial initiatives for COVID-19. For instance, in July 2023, the National Institutes of Health introduced the RECOVER Initiative, targeting long-COVID clinical trials. Thus, the demand for equipment and ancillary solutions is expected to increase significantly with rising number of initiatives.
As the number of clinical trials increases, more resources are needed for conducting those trails, including equipment, ancillary supplies, and drugs. Ancillary supplies comprise all objects essential to conduct a study, such as surgical knives, syringes, swabs, gloves, and any other entities that patients and medical personnel require to administer medications and examine the efficacy and safety parameters under investigation.
Sourcing instruments & ancillary objects for clinical trials involve more than just procurement. It requires an in-depth knowledge of the study’s design and prerequisites, as well as relentless & proactive planning to ensure that the most pertinent tools and supplies are purchased at the proper time and an appropriate price. A combination of internal knowledge and external help is suggested in managing intricacies of equipment and ancillary supply, logistics, restocking, and expiry management.
Additionally, increased investments in R&D and the adoption of virtual clinical trials due to the COVID-19 pandemic have boosted industry growth. Furthermore, there is a tremendous upsurge in the number of studies undertaken for COVID-19, which is anticipated to enhance industry growth. For instance, according to an article by the U.S. Department of Health & Human Services in September 2023, the Strategies and Treatments for Respiratory Infections and Viral Emergencies (STRIVE), part of the global clinical trials consortium, would register about 1,500 individuals at research locations globally to test immune modulation method for hospitalized COVID-19 patients. Such trials and a growing interest of industry participants in clinical studies are expected to boost growth in the coming years.
Market growth stage is high, and the pace of the market growth is accelerating. The clinical trial equipment & ancillary solutions industry is characterized by a moderate degree of innovation due to rapid technological advancements, such as adoption of machine learning and AI in clinical trial platforms. Companies are entering the market and bringing novel technologies and platforms, which is expected to drive industrial innovations.
For instance, in November 2023, pharmaceutical giant AstraZeneca introduced the health technology business Evinova to enhance clinical trial processes. It offers digital technology tools to streamline clinical trial design and delivery to decrease time and costs in medicine development. This business would utilize artificial intelligence and machine learning to help clinical teams craft optimal studies. Furthermore, in October 2023, H1 introduced GenosAI, a new generative AI tool implanted into Trial Landscape, the compnay’s clinical trial intelligence platform, to scrutinize and respond to complicated queries.
The market is characterized by a moderate level of merger and acquisition (M&A) activities among leading players. This is due to several factors, including the desire to gain a competitive advantage in this industry and the need to consolidate in a rapidly growing market. Major industry participants are acquiring firms providing advanced services and solutions for clinical studies.
For instance, in October 2022, Myonex, a major clinical trial supply organization, acquired the clinical trial and packaging business of the Germany-based organization, Hubertus Apotheke. This acquisition is expected to support the capabilities and resources for both sourcing and packaging, including decentralized trials. Such acquisitions are expected to propel industry growth in the coming years.
Furthermore, industry participants are focusing on increasing their presence in several regions and improving access to instruments needed during clinical studies globally. For instance, in December 2022, Catalent announced the completion of the expansion of its clinical supply facility in Shanghai, China. With this expansion, the company increased the facility by about 30,000 sq. ft., adding refrigerated and deep-frozen storage and extended secondary packaging capacities. Thus, regional expansions by the major players are expected to improve access to these products.
The supply/logistics segment accounted for a dominant revenue share of 40.0% in 2023. As clinical trials have become more global and diverse, they require a proper cold-chain logistics system to maintain the integrity of temperature-sensitive drugs. Fluctuation in rules & regulations in emerging markets is expected to boost the need for supply/logistic services during the forecast period. Furthermore, presence of companies such as AKESA PTY LTD, Movianto, Parexel International (MA) Corporation., and Oximio offering supply and logistics solutions is expected to propel segment growth in the coming years.
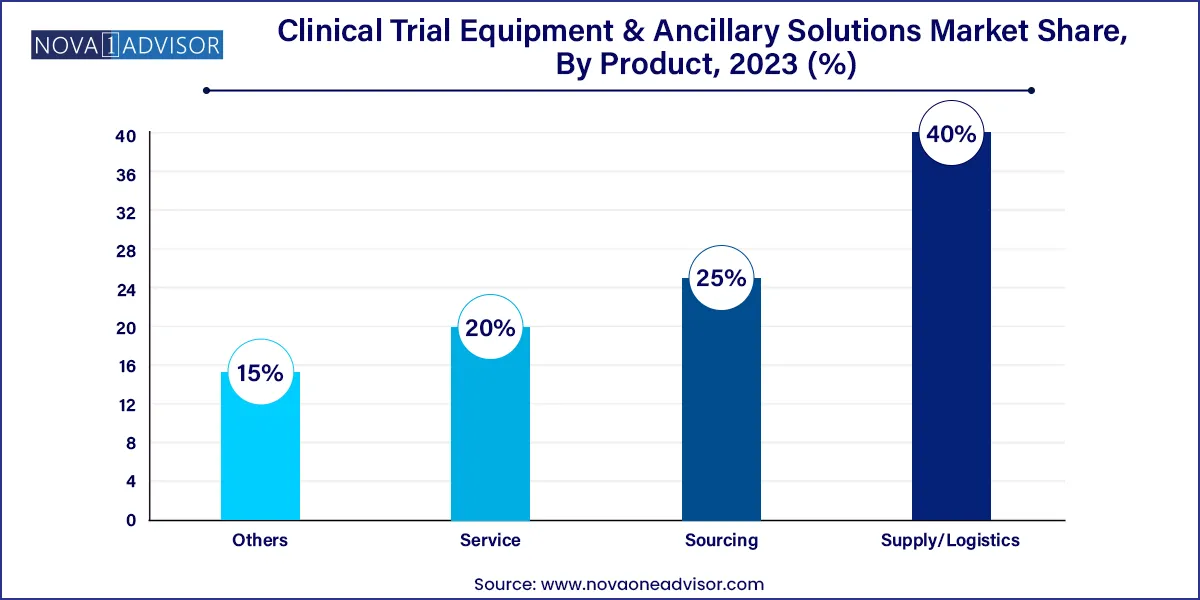
The sourcing segment is anticipated to witness strong growth through 2033. Owing to the rising intricacy of clinical trials, the quantity of tools and ancillary supplies needed, as well as the number of suppliers required has risen substantially. The challenge of ensuring that supplies reach sites in a cost-effective manner for worldwide research has increased. With sourcing and distribution expertise, there is a single point of contact to supply and transport equipment and ancillary supplies worldwide. This streamlined and coordinated procedure removes the strain on teams conducting trials and facilities.
The phase III segment dominated the market in 2023 and is expected to maintain its dominance through 2033. Investigations in phase III are more complex than those in earlier phases. Even though the number of medications in this phase is relatively minimal, intricacies associated with this stage are immense. In addition, failure rates are also more as the research design and sample size require accurate dosing at an ideal level. This leads to human and economic damage, and most failures are generated by non-compliance with safety provisions. Such a scenario is likely to boost the demand for effective supply chain and logistics, which is projected to boost market growth over the coming years.
On the other hand, the phase I segment is projected to witness the highest growth rate over the forecast period due to the increasing number of pharmaceutical companies that are focusing on the development of novel therapeutics for rare diseases. Growth in the number of CROs globally is another factor supporting the adoption of phase I clinical trials to boost the rapid development of drugs.
North America dominated the clinical trial equipment & ancillary solutions market with a 40.15% revenue share in 2023. This dominance is attributed to the fact that most pharmaceutical businesses are located in the U.S. and perform most of their business in this region. Favorable regulatory policies, the introduction of technologically advanced products by market players, and increasing investments by pharmaceutical firms are expected to drive regional market growth during the forecast period. Companies operating in this industry are expanding their presence in the U.S. For instance, in March 2023, Almac Group invested USD 65 million to expand its supply-chain services for clinical trials in Montgomery County, Pennsylvania. Such expansions are expected to boost regional growth.
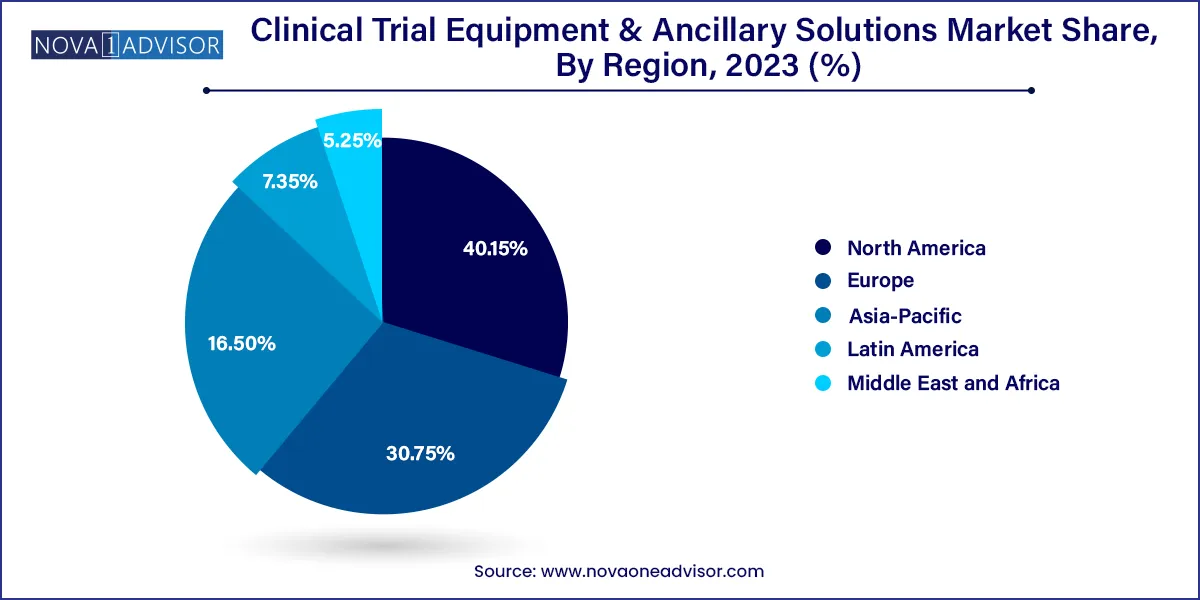
Asia Pacific is anticipated to witness fastest growth in the clinical trial equipment and ancillary solutions market. Asia Pacific has become a hotspot for performing clinical trials on account of inexpensive study costs, ease of regulatory compliance, an increasing patient population in this region, and the increasing presence of elite clinical institutions that are functioning as sites.
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the global clinical trial equipment & ancillary solutions market.
Product
Phase
By Region