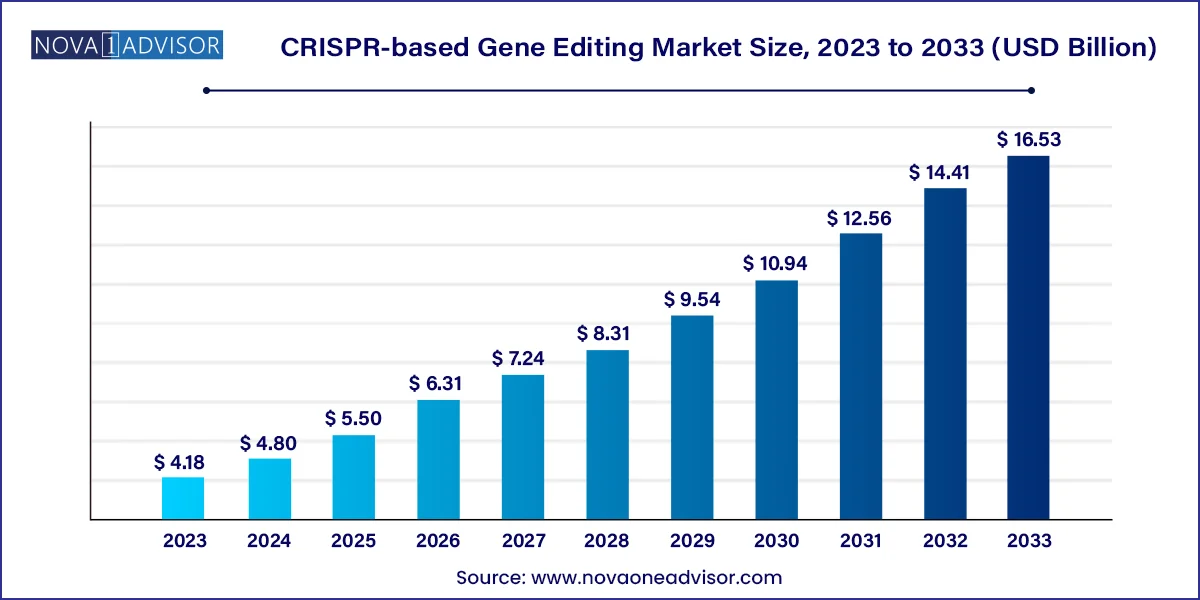
The global CRISPR-based gene editing market size was valued at USD 4.18 billion in 2023 and is anticipated to reach around USD 16.53 billion by 2033, growing at a CAGR of 14.74% from 2024 to 2033.
The CRISPR-based gene editing market represents a groundbreaking transformation in molecular biology, biomedical innovation, and biotechnology applications. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology, particularly CRISPR-Cas9, has redefined the potential of genetic modification by offering a highly accurate, cost-effective, and programmable system for editing DNA. Since its first major application in mammalian systems in 2013, CRISPR has rapidly evolved from a revolutionary laboratory tool to a clinically relevant platform with vast applications ranging from disease therapy and agriculture to diagnostics and synthetic biology.
This market comprises a broad ecosystem of product developers, therapeutic biotech companies, research institutions, and service providers. CRISPR-based technologies are being developed for both somatic and germline gene modifications, although clinical and ethical frameworks currently limit the latter. The recent regulatory approval of the world’s first CRISPR-based therapy, Casgevy, for sickle cell disease and beta thalassemia in the U.K. and U.S. marks a major inflection point for commercialization.
CRISPR’s role in therapeutic development, particularly for monogenic disorders, oncology, and ophthalmology, has driven significant investor interest and clinical pipeline expansion. Additionally, CRISPR’s integration into diagnostics (via SHERLOCK, DETECTR platforms) and agriculture (for yield improvement and stress tolerance) extends the market’s influence beyond therapeutics. With the convergence of CRISPR technologies, AI-driven gene design, and delivery innovations, the global CRISPR market is entering a new phase of clinical utility and commercial scalability.
First FDA-approved CRISPR-based therapy marks clinical maturity (Casgevy for sickle cell, Dec 2023).
Expansion into multiplex editing and base editing technologies for precision and reduced off-target effects.
Rising partnerships between CRISPR startups and pharmaceutical giants for co-developing gene therapies.
Integration with AI and machine learning for guide RNA design, target prediction, and off-target analysis.
Surging investment in ex vivo CRISPR-based cell therapies, particularly in oncology.
CRISPR-Cas12 and Cas13 systems expanding into molecular diagnostics for infectious diseases.
Ethical and regulatory frameworks are evolving, enabling phased entry into broader clinical indications.
Adoption in agrigenomics: Developing disease-resistant crops and genetically edited livestock gaining regulatory approval in several countries.
| Report Attribute | Details |
| Market Size in 2024 | USD 4.80 Billion |
| Market Size by 2033 | USD 16.53 Billion |
| Growth Rate From 2024 to 2033 | CAGR of 14.74% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Product & Service, application, end use, region |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Key Companies Profiled | Revvity, Inc.; Danaher Corporation; GenScript; Merck KGaA; Thermo Fisher Scientific, Inc.; Tocris Bioscience; OriGene Technologies, Inc.; Bio-Rad Laboratories; Bio-Techne; New England Biolabs, Inc. |
One of the most pivotal drivers of the CRISPR gene editing market is the successful translation of CRISPR-based therapeutics from lab to clinic, culminating in regulatory approvals. In December 2023, the U.S. FDA and the UK’s MHRA approved Casgevy (exagamglogene autotemcel)—a CRISPR/Cas9-based gene therapy co-developed by Vertex Pharmaceuticals and CRISPR Therapeutics—for treating sickle cell disease and transfusion-dependent beta thalassemia. This milestone, resulting from a decade of clinical and preclinical validation, demonstrated that CRISPR is not merely a research tool but a commercially viable therapeutic platform.
Such validation has amplified confidence among investors, biotech firms, and regulatory bodies. Multiple clinical-stage companies are now progressing trials for conditions such as Leber congenital amaurosis, hereditary angioedema, and several cancers. The regulatory success of Casgevy opens the door to broader use across other monogenic and multifactorial diseases, significantly accelerating the overall market growth trajectory.
Despite the remarkable scientific promise, the CRISPR-based gene editing market faces regulatory and ethical constraints, particularly regarding off-target effects, germline editing, and long-term patient safety. While ex vivo therapies offer more controlled environments, in vivo editing still presents risks of unintended genetic alterations. This has led to cautious regulatory oversight, with stringent requirements for data transparency, trial design, and post-treatment monitoring.
Ethical debates over human germline editing—especially after the controversial birth of CRISPR-edited babies in 2018—have prompted international regulatory bodies to adopt a conservative stance. Additionally, the complex intellectual property (IP) landscape surrounding CRISPR-Cas technologies, notably the ongoing legal disputes between the Broad Institute and University of California, Berkeley, has also created uncertainty for market participants regarding licensing and commercialization rights.
While biomedical applications dominate public discourse, the agricultural sector presents a highly scalable and underexplored opportunity for CRISPR-based innovations. Gene editing in plants and farm animals offers the potential to address food security, disease resistance, and environmental sustainability. Crops edited using CRISPR for drought tolerance, fungal resistance, or enhanced nutrition are entering regulatory pipelines and commercial fields in regions such as the U.S., Brazil, and China.
CRISPR-engineered livestock with improved productivity and disease resistance traits (e.g., hornless cattle, porcine reproductive and respiratory syndrome virus–resistant pigs) are also under investigation. Unlike GMOs, CRISPR-edited organisms with targeted, non-transgenic edits are facing less regulatory resistance in certain jurisdictions, making them commercially viable in shorter time frames. This sector offers a complementary revenue stream for CRISPR developers and diversifies the market beyond therapeutics.
CRISPR kits and reagents currently dominate the market, owing to their indispensable role in R&D workflows across academic, pharmaceutical, and biotechnology institutions. These products include guide RNAs, Cas nucleases, delivery systems, and editing validation tools, all of which are required for preclinical studies and experimental setups. The consistent demand from research laboratories and the rapid adoption of newer CRISPR variants (e.g., Cas12, Cas13, base editors) ensures strong growth for this segment.
Suppliers like Thermo Fisher, Agilent, and Integrated DNA Technologies offer customizable and off-the-shelf kits that simplify CRISPR workflows, enabling broad accessibility. As CRISPR research continues to diversify into synthetic biology, diagnostics, and drug discovery, the demand for these foundational reagents remains resilient.
CRISPR-based gene editing services—covering guide RNA design, cell line generation, therapeutic vector development, and screening—are the fastest growing subsegment, driven by outsourcing trends and the complexity of CRISPR-based experimentation. Biopharma companies and academic labs are increasingly relying on service providers to reduce the time and cost associated with developing and validating gene editing protocols.
Contract research organizations (CROs) and specialized service companies offer high-throughput screening, off-target analysis, and custom library construction, enabling clients to accelerate therapeutic pipelines. As more players enter clinical development, service demand is expected to expand rapidly, particularly for GMP-grade solutions and IND-enabling studies.
Therapeutic development remains the dominant application, accounting for the highest share of CRISPR market revenues. The segment includes CRISPR applications in treating monogenic disorders, hematological conditions, cancers, and eye diseases. Ex vivo gene editing in hematopoietic stem cells has demonstrated strong safety and efficacy profiles in treating sickle cell disease, beta thalassemia, and certain leukemias.
The therapeutic promise of in vivo CRISPR therapies is also gaining traction, with companies like Editas Medicine and Intellia Therapeutics advancing clinical programs in inherited retinal diseases and transthyretin amyloidosis. The potential for one-time, curative treatment makes CRISPR therapies highly attractive to both patients and payers, reinforcing the dominance of this segment.
Agricultural applications are emerging as the fastest growing segment, fueled by increasing global demand for food security, climate-resilient crops, and disease-resistant livestock. CRISPR is being applied in rice, maize, wheat, soy, and tomatoes to improve yield, enhance nutrient profiles, and develop herbicide resistance. Regulatory frameworks in the U.S., Japan, and Argentina have created favorable pathways for non-transgenic edited crops, accelerating commercialization.
Additionally, the potential to edit livestock genomes without foreign DNA insertion presents new avenues in sustainable agriculture. As public and regulatory acceptance increases and R&D pipelines mature, CRISPR’s impact in agrigenomics is poised to accelerate.
Based on end use, the pharmaceutical & biotechnology companies segment led the market with the largest revenue market share of 46.51% in 2023. Primarily due to their central role in therapeutic product development and clinical translation. These organizations invest heavily in CRISPR research to build gene therapy pipelines, secure intellectual property, and enter licensing or collaboration agreements. Companies like CRISPR Therapeutics, Intellia Therapeutics, and Beam Therapeutics have raised billions in funding and built advanced CRISPR drug development platforms.
Moreover, Big Pharma is partnering or acquiring CRISPR startups to access expertise, IP, and platform technologies. The involvement of biopharma ensures a steady flow of investment, regulatory engagement, and commercial readiness for gene editing solutions.
Academic and research institutes remain the fastest growing contributors, especially in foundational CRISPR research, tool development, and proof-of-concept disease models. Many novel CRISPR applications—including epigenome editing, prime editing, and synthetic gene circuits—originate from academia. NIH-funded programs, the Innovative Genomics Institute, and various university spin-offs continue to push the boundaries of CRISPR science.
Academic centers also play a vital role in early-stage discovery, rare disease research, and collaboration with biotech companies for clinical translation. As government and philanthropic funding into CRISPR increases, this segment will continue to grow in importance and output.
North America Dominated the Market
North America is the global leader in the CRISPR gene editing market, driven by the presence of key players, strong academic institutions, advanced healthcare infrastructure, and a supportive regulatory environment. The U.S. houses major CRISPR developers like CRISPR Therapeutics, Intellia, Editas Medicine, and Beam Therapeutics, many of which are conducting FDA-sanctioned trials. The region also leads in venture capital funding and intellectual property generation related to CRISPR technologies.
Support from organizations like the National Institutes of Health (NIH), along with regulatory transparency from the FDA, has fostered a robust innovation ecosystem. North America is also a leading adopter of CRISPR in agriculture and diagnostics, making it the most diversified and commercially mature regional market.
Asia-Pacific Is the Fastest Growing Region
Asia-Pacific is the fastest growing region, propelled by significant investment in biotechnology, growing clinical trial activity, and government-backed genomic research initiatives. Countries like China, Japan, South Korea, and Singapore are expanding CRISPR applications in agriculture, regenerative medicine, and oncology. China, in particular, is leading CRISPR crop development and has multiple trials underway for CRISPR-edited therapies.
The region’s large patient populations, growing biotech sector, and relatively flexible regulatory frameworks in agriculture create a conducive environment for rapid market expansion. Asia-Pacific’s strategic role in CRISPR production, clinical testing, and application diversification positions it for continued high growth through 2034.
In March 2025, Beam Therapeutics announced positive preclinical data for its base editing program targeting sickle cell disease, using next-gen CRISPR tools with reduced off-target effects.
In January 2025, Intellia Therapeutics received FDA Fast Track designation for its in vivo CRISPR therapy for hereditary angioedema, highlighting growing regulatory support.
In November 2024, Corteva Agriscience received USDA clearance for a CRISPR-edited drought-tolerant soybean, advancing CRISPR’s agricultural applications in the U.S.
In October 2024, Editas Medicine launched a partnership with BMS to co-develop allogeneic CAR-T therapies using CRISPR for solid tumors.
In August 2024, CRISPR Therapeutics and Vertex Pharmaceuticals secured FDA approval for Casgevy, marking the first approved CRISPR-based therapy in the U.S.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the CRISPR-based Gene Editing market.
By Product & Service
By Application
By End Use
By Region