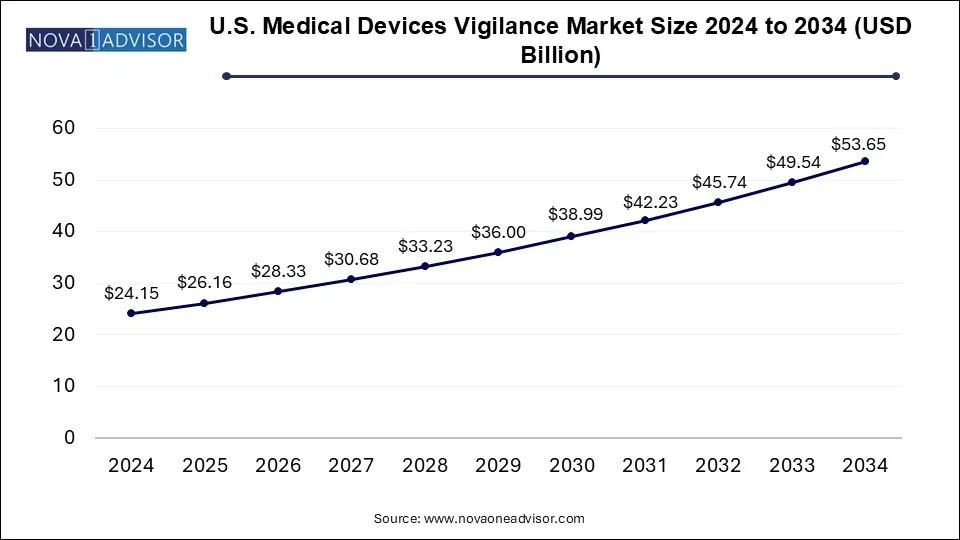
The medical devices vigilance market size was exhibited at USD 87.35 billion in 2024 and is projected to hit around USD 192.28 billion by 2034, growing at a CAGR of 8.21% during the forecast period 2025 to 2034. Instances of device failures prompting investment in vigilance systems to prevent future incidents with the safety of patients is the prime purpose of the healthcare system.
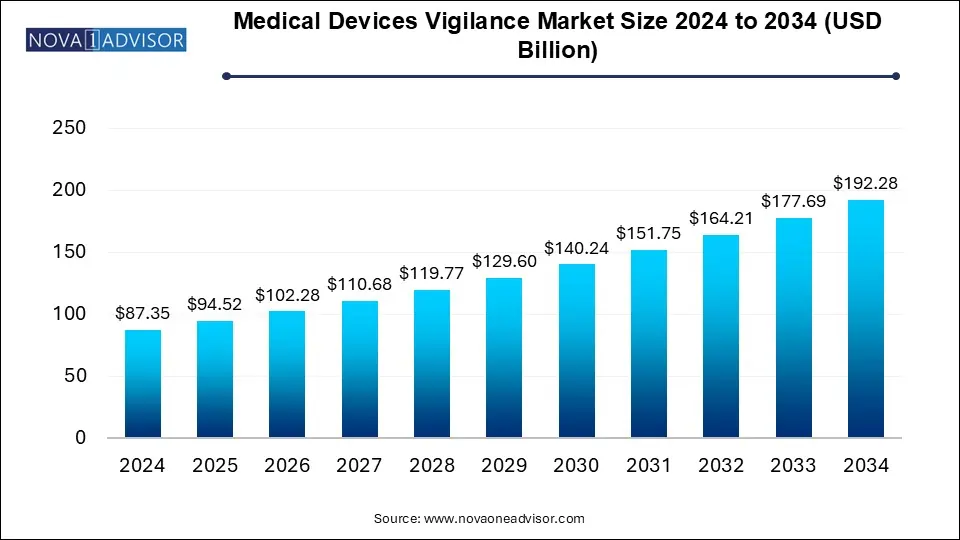
The U.S. medical devices vigilance market size surpassed USD 24.15 billion in 2024 and is expected to be worth around USD 53.65 billion by 2034 at a CAGR of 8.31% from 2025 to 2034.
North America is projected to command a significant share of the medical devices vigilance market, presenting a promising landscape marked by strict regulatory policies, widespread adoption of medical devices, and a strong culture of technological advancements. These factors collectively contribute to the region’s sustained market growth and innovation.
The medical devices vigilance sector in North America is poised for substantial expansion due to several critical drivers. Firstly, the region benefits from a well-established healthcare infrastructure, with state-of-the-art medical facilities and a high adoption rate of medical devices. The extensive use of medical equipment ranging from implantable devices to diagnostic tools fuels the necessity for comprehensive vigilance systems to uphold patient safety. Secondly, the regulatory landscape in the United States and Canada mandates rigorous monitoring and reporting of adverse events related to medical devices, ensuring stringent compliance with safety standards.
Moreover, North America leads in technological innovation, with a growing focus on integrating artificial intelligence, machine learning, and big data analytics into healthcare solutions. This presents a significant opportunity for companies within the medical devices vigilance industry to develop advanced monitoring solutions that improve safety and operational efficiency.
Asia Pacific: The Fastest-Growing Region in Medical Devices Vigilance
The Asia Pacific region is experiencing rapid expansion in the medical devices vigilance market, driven by several key factors. One of the primary contributors is the rising demand for healthcare services in high-population countries such as China, India, and Japan, which is accelerating the adoption of medical devices across multiple medical specialties.
With the increasing reliance on medical devices, awareness regarding their safety and effectiveness has also risen, resulting in heightened demand for vigilance solutions. Additionally, regulatory agencies in the region are strengthening oversight of medical device safety and surveillance. Countries like China and India are enhancing their regulatory frameworks to align with international best practices, enforcing stricter monitoring and reporting requirements for adverse events. This regulatory shift is driving the need for comprehensive vigilance systems, creating opportunities for companies specializing in medical device surveillance.
India has introduced a Materiovigilance Programme of India (MvPI) to systematically collect and analyze data on adverse events related to medical devices. This initiative plays a crucial role in assisting regulatory authorities in making informed decisions and ensuring the safe usage of medical devices.
Furthermore, Asia Pacific is undergoing rapid digital transformation and technological advancements in healthcare, creating an ideal environment for innovation in medical device vigilance. Companies are increasingly utilizing AI-powered analytics, cloud computing, and big data solutions to develop efficient monitoring systems that track device performance and detect potential safety risks.
Thus, the Asia Pacific region represents a highly dynamic and rapidly expanding market for medical device vigilance, driven by rising healthcare needs, evolving regulatory frameworks, and significant technological progress. This market presents vast opportunities for growth and investment, making it an attractive segment for companies operating in this space.
| Report Coverage | Details |
| Market Size in 2025 | USD 94.52 Billion |
| Market Size by 2034 | USD 192.28 Billion |
| Growth Rate From 2025 to 2034 | CAGR of 8.21% |
| Base Year | 2024 |
| Forecast Period | 2025-2034 |
| Segments Covered | By Delivery Mode, By Application, and By End-user |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope | North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled | ZEINCRO, AssurX Inc., Sparta System, Oracle Corporation, Xybion Corporation, Sarjen Systems Pvt. Ltd., MDI Consultants, Inc., AB-Cube, Laerdal Medical., Omnify Software, Inc., and Others. |
The medical devices vigilance market plays a crucial role in monitoring the safety and performance of medical equipment and tools. It acts as a protective system, ensuring that devices function correctly and do not pose risks to patients. Essentially, it serves as a safeguard for medical devices, helping to prevent malfunctions and adverse events.
The market is experiencing significant growth due to the increasing use of medical devices, such as pacemakers, insulin pumps, and other critical healthcare technologies. As the reliance on these devices grows, so does the need to ensure their safety and efficiency. For example, if a pacemaker fails unexpectedly, it could result in serious complications. To mitigate such risks, companies are heavily investing in vigilance systems that can detect potential failures and improve patient safety.
The rising demand for reliable and secure medical interventions is a key driver of market expansion. Since any errors in device functionality can be life-threatening, even when operated by skilled healthcare professionals, medical device vigilance has become essential. It reinforces patient safety, builds trust in medical technology, and ultimately propels the rapid growth of this market.
Medical Devices Vigilance Market Growth Factors
Medical Devices Vigilance Market Dynamics
Driver: The Need for Continuous Monitoring
The growing dependence on medical devices worldwide has heightened the necessity for constant monitoring. As technological advancements lead to more sophisticated devices for diagnosing, treating, and tracking various health conditions, ensuring their safety and efficacy becomes crucial. Devices such as pacemakers, insulin pumps, implantable defibrillators, and continuous glucose monitors play a vital role in patient care, especially for those managing chronic diseases.
However, with the increasing usage of these medical technologies, concerns regarding device safety and performance have risen. Malfunctions or failures can have severe consequences, including injury or fatal outcomes. In response, regulatory authorities are enforcing stricter monitoring and reporting standards to enhance patient safety.
A study published in JAMA Internal Medicine highlighted that 23% of reported serious injury or death cases were incorrectly classified and not attributed to defective medical devices. This misreporting accounted for nearly 31,500 preventable deaths, underscoring the critical need for a robust vigilance system to implement corrective and preventive actions effectively.
Furthermore, both healthcare providers and patients are becoming increasingly aware of the importance of device vigilance in maintaining patient safety. They seek assurance that the medical devices they rely on are not only effective but also reliable and free from defects. As a result, the demand for vigilance solutions and compliance services is growing, making device safety concerns a significant driver of market expansion on a global scale.
Restraint: Complexity and High Costs
One of the major challenges in the medical devices vigilance market is the complexity and high costs involved in implementing monitoring and compliance systems. Establishing and maintaining an effective vigilance framework requires substantial investments in technology, skilled personnel, and infrastructure.
Additionally, medical device regulations are constantly evolving, imposing stringent compliance requirements. Companies must navigate a complicated regulatory landscape while adhering to strict reporting obligations. This is particularly challenging for smaller enterprises with limited financial and operational resources, making it difficult for them to keep pace with evolving regulatory standards.
Moreover, some stakeholders within the healthcare sector may view vigilance protocols as cumbersome and disruptive to their operations. Resistance to these measures can hinder widespread adoption, delaying the implementation of critical safety improvements. Consequently, while the demand for vigilance solutions is on the rise, these obstacles could slow down overall market growth and create challenges for businesses aiming to expand their presence in the sector.
Opportunity: Adoption of Advanced Technologies
The integration of advanced technologies, including artificial intelligence (AI) and machine learning (ML), presents a significant growth opportunity in the medical devices vigilance market. AI and ML can be used to analyze large datasets, identify patterns in adverse event reports, and enable early detection of safety issues. This proactive approach allows manufacturers to take preventive actions before device failures result in harm.
Moreover, AI-driven predictive analytics can improve risk assessment by offering personalized monitoring strategies based on individual patient profiles, including medical history, genetic factors, and environmental influences. This personalized vigilance not only enhances patient safety but also leads to better healthcare outcomes.
Additionally, AI and automation can streamline regulatory compliance by reducing the administrative burden associated with documentation, reporting, and audits. By automating compliance processes, companies can minimize costs, improve efficiency, and focus on innovation.
Thus, the integration of AI and digital technologies in medical device vigilance is set to transform the industry, enabling smarter monitoring systems, improved safety standards, and regulatory efficiency, ultimately driving sustained market growth.
The on-demand segment held the largest market share of 82.0% in 2024 within the medical devices vigilance market. This segment plays a crucial role in ensuring quick access to essential medical devices, particularly for urgent medical situations and emergencies. By utilizing advanced technology and efficient logistics, on-demand delivery optimizes the distribution process, ensuring that medical devices reach healthcare providers and patients promptly. The ability to minimize delays and maintain a steady supply of critical medical equipment significantly enhances patient care and safety.
Meanwhile, the on-premises segment contributed to 18.0% of total revenue in 2024 and is expected to maintain steady growth over the coming years. The on-premises model allows healthcare facilities to exercise greater control over vigilance operations by managing them within their infrastructure. This approach provides the flexibility to customize monitoring protocols to meet institution-specific needs while ensuring compliance with regulatory standards and data protection laws. Additionally, in-house vigilance enables faster responses to potential device issues, allowing healthcare providers to quickly address safety concerns and uphold patient well-being.
In 2024, the diagnostics segment held a significant market share in the medical devices vigilance sector. This segment leverages advanced diagnostic technologies to monitor device functionality and detect potential failures in real time. By employing specialized algorithms and data analysis tools, it helps assess device performance, detect irregularities, and notify healthcare providers of necessary interventions. This proactive strategy enhances device reliability, reduces adverse events, and strengthens patient safety. Through continuous surveillance and diagnostic assessments, this segment plays a vital role in maintaining medical device integrity across the healthcare system.
The research segment is forecasted to witness substantial growth in the coming years. This segment is integral to enhancing knowledge about medical device safety and efficiency. Researchers conduct clinical trials, observational studies, and post-market surveillance to evaluate real-world device performance. Their findings contribute to regulatory improvements, technological advancements, and patient safety enhancements. By providing critical insights to manufacturers, healthcare providers, and government agencies, the research segment drives innovation and ensures continuous improvements in medical device vigilance.
In 2024, the clinical research organization (CRO) segment accounted for the largest market share in the medical devices vigilance market. CROs specialize in conducting clinical studies, monitoring device performance, and ensuring regulatory compliance for medical device manufacturers and governing bodies. Their expertise in data collection, risk assessment, and reporting makes them indispensable in evaluating device safety and efficacy throughout the product lifecycle. By collaborating with CROs, medical device companies can expedite regulatory approvals, refine device performance, and accelerate product commercialization. Given their robust infrastructure and skilled workforce, CROs remain key players in advancing medical device vigilance and innovation.
The business process outsourcing (BPO) firms segment is anticipated to experience rapid expansion in the near future. BPO firms provide specialized vigilance services, including adverse event reporting, complaint management, and regulatory compliance assistance. By outsourcing these functions, medical device companies can optimize operational efficiency, lower costs, and gain access to specialized expertise that may not be available in-house. The BPO sector offers scalable and tailored solutions, enabling manufacturers to focus on their core business functions while ensuring adherence to stringent regulatory requirements. As the demand for efficient and cost-effective vigilance processes continues to grow, the BPO segment is expected to witness sustained market expansion.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the medical devices vigilance market
By Delivery Mode
By Application
By End-user
By Regional