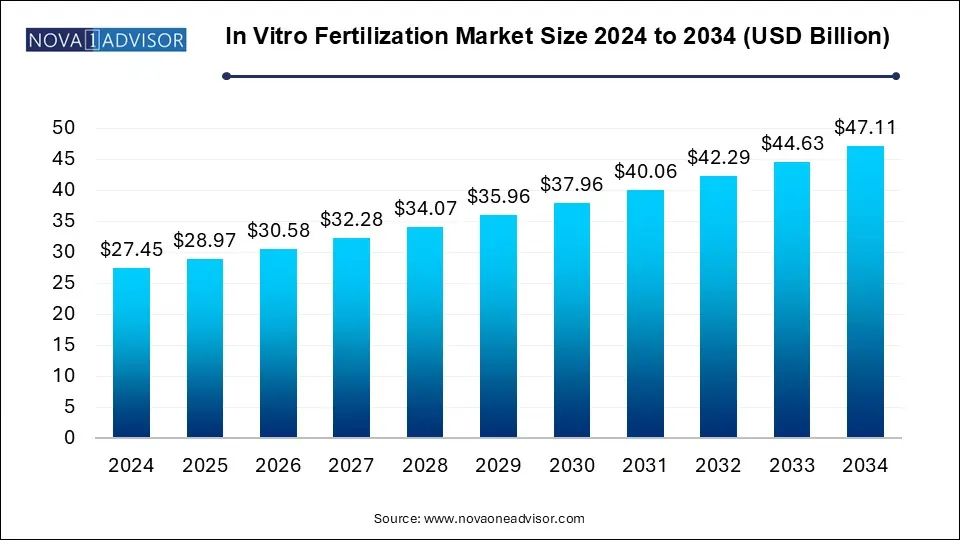
The In vitro fertilization market size was exhibited at USD 27.45 billion in 2024 and is projected to hit around USD 47.11 billion by 2034, growing at a CAGR of 5.55% during the forecast period 2025 to 2034.
| Report Coverage | Details |
| Market Size in 2025 | USD 28.97 Billion |
| Market Size by 2034 | USD 47.11 Billion |
| Growth Rate From 2025 to 2034 | CAGR of 5.55% |
| Base Year | 2024 |
| Forecast Period | 2025-2034 |
| Segments Covered | Instrument, Procedure Type, Providers, Region |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope | North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled | Bayer AG; Cook Medical LLC; EMD Serono, Inc.; Ferring B.V.; FUJIFILM Irvine Scientific (FUJIFILM Holdings Corporation); Genea Biomedx; EMD Serono, Inc. (Merck KGaA); Merck & Co., Inc.; The Cooper Companies, Inc.; Thermo Fisher Scientific, Inc.; Vitrolife; Boston IVF; Nova IVF; RMA Network (Reproductive Medicine Associates); TFP Thames Valley Fertility; Fortis Healthcare; U.S. Fertility |
Rising reproductive tourism and increasing cases of male & female infertility are key factors driving the market growth. Infertility is one of the major health concerns faced by individuals globally. According to WHO, around 17.5% of the adult population worldwide experiences infertility. This shows necessity to enhance accessibility to affordable and top-notch fertility care globally.
The American Pregnancy Association states that male infertility constitutes 30% of overall infertility cases and contributes to approximately one-fifth of all infertility instances in the U.S. The average age at which individuals are getting married and having their first child is increasing. This trend has increased number of women seeking IVF treatment. Moreover, to focus on their career, many women freeze their eggs to have child at a later stage. Rising dependence on fertility treatments is expected to aid market growth. The availability of funds is leading to a rise in adoption of in vitro fertilization (IVF) procedures.
To increase success rate of IVF, techniques, such as egg/sperm freezing, vitrification, assisted hatching, Percutaneous Epidydimal Sperm Aspiration & Testicular Sperm Extraction (PESA and TESE), are being introduced along with development of new products. In May 2024, AIVF Ltd., a company based in field of AI-based solutions for IVF clinics, joined forces with Genea Biomedx, a medical device provider for IVF laboratories, to create a comprehensive and cost-effective integrated systems solution for personalized IVF. The collaboration brought together Genea Biomedx’s Geri time-lapse incubator and AIVF Ltd.’s EMA AI platform, creating a powerful suite that offers widespread access to personalized and optimized IVF treatments.
To compete in modern business environment, all organizations (manufacturers, clinics, and hospitals) must develop their virtual presence to increase awareness about infertility treatment, and their services. As infertility is a sensitive issue, people are reluctant to discuss it openly, particularly in developing countries. Hence, developing authenticity and trust through digital platforms is a key challenge for service providers. The providers can take an initial step to gain a center’s or manufacturer’s trust by displaying hospital’s or approval committee’s certificates, introducing doctors or scientists, and sharing their coordinates to establish & authenticate their identity.
The culture media segment dominated the market with the largest revenue share of 40.9 % in 2024. This can be attributed to factors such as availability of funding and an increase in research activities to improve culture media. In July 2022, FUJIFILM Irvine Scientific launched a mineral oil for embryo culture, Heavy Oil for Embryo Culture. It is a sterile mineral oil that addresses major concerns in IVF procedures, including pH, osmolality changes, and media preservation, owing to its ideal weight viscosity.
Disposable devices segment is expected to grow at the fastest CAGR during forecast years. The growth of this segment is owing to industry players introducing disposable devices, such as slides, needles, and chambers, to meet sterility and regulatory requirements. Such developments are expected to increase adoption of disposable IVF devices. Disposable slides for sperm counting, an imaging-based tracking system to isolate best motile sperm, and use of disposable microchips are some of innovations witnessed by the market in recent years.
Frozen non-donor segment dominated the market with largest revenue share in 2024 and is expected to witness fastest growth over the forecast period. Certain factors contributing to high share are cost-effectiveness as compared to fresh nondonor and less invasive nature of procedure. The fresh donor segment is expected to grow significantly over forecast period. As per the 2020 National ART Summary report, around 1,477 ART cycles were performed using fresh donors, with around 53.9% of transfers resulting in live-birth deliveries in the U.S.
Although some centers offer risk-sharing plans and refunds only for three cycles, Advanced Fertility Center of Chicago has designed IVF reimbursement plan to provide a 100% refund for up to four cycles with fresh embryos. The Human Fertilization and Embryology Act 1990 does not cover fresh sperm donation, and hence, there is rising concern about sperm donors not being screened before donation. HEFA does not guarantee sperm donation services, which are unlicensed. Hence, HEFA has revised its guidelines, wherein fresh sperm must be quarantined for 180 days for HIV screening. NHS funds a smaller proportion of fertility treatment using donated gametes to homosexuals and single parents.
Fertility clinics segment dominated the market with largest revenue share of 80.0% in 2024 and is expected to witness fastest growth over the forecast period. This can be attributed to a rise in demand for ART treatments; number of fertility clinics and ART centers is increasing considerably. Factors, such as cost-effectiveness, availability of specialists, and minimal or no chances of Hospital-Acquired Infections (HAIs), are anticipated to drive the growth of fertility clinics segment. IVF treatments are also performed in hospitals.
Hospitals and other settings segments is expected to grow at a significant CAGR over the forecast period. Numerous multispecialty hospitals provide infertility treatments, including In-vitro Fertilization (IVF). Growing accessibility and availability of these treatments have contributed to a greater inclination toward hospitals for infertility care. However, hospital IVF treatments cost more than fertility clinics. This is partly due to need for highly skilled physicians and staff to perform these intricate procedures. Consequently, having a dedicated IVF staff in hospitals is considered a less favored approach due to expenses related to their employment, remuneration, and training. These costs can be particularly high in developed countries like the U.S. and UK.
Europe dominated the market with largest revenue share of 37.0% in 2024. This can be attributed to factors, such as a noticeable surge in medical tourism, with an increasing number of Americans choosing to travel to Czech Republic for more cost-effective IVF treatments. Moreover, individuals who cannot afford to travel abroad are now seeking IVF treatments within the United States at around one-third of the cost charged by clinics in the country. In July 2022, Fairtility obtained approval from the European Union to use AI for embryo assessment. EU's new medical device regulation standards have enabled development of a commercially available AI tool, which holds potential to boost success rate of IVF procedures.
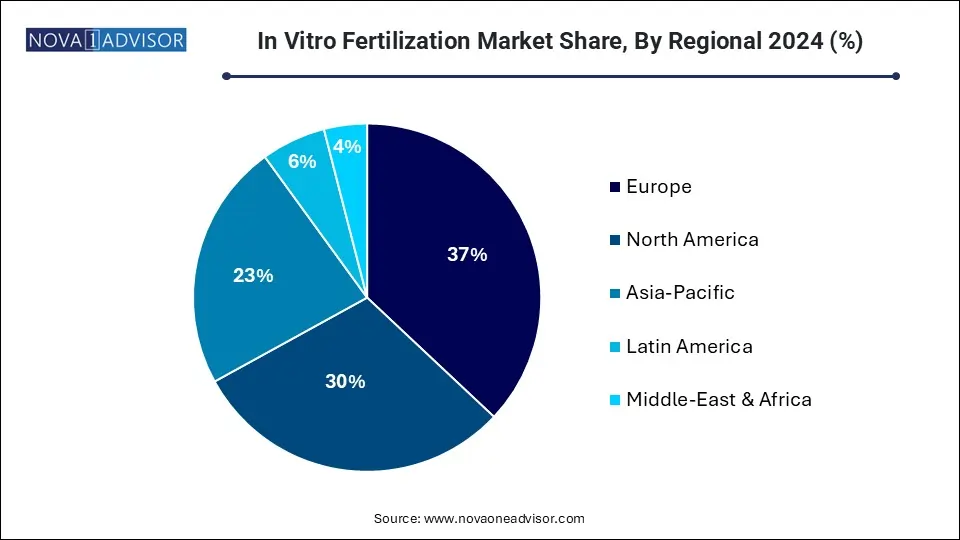
North America is also expected to witness increasing demand for fertility treatment in coming years. Lifestyle changes, including insufficient nutrition, stress, increased obesity, improper eating habits, rise in pollution, lack of exercise, and prevalence of medical conditions like diabetes, have led to a higher incidence of infertility in the region. The growth of can be attributed to several factors such as standardization of procedures through regulatory reforms, automation, government funding for egg/sperm storage, and introduction of more IVF treatments by industry players.
U.S. IVF Market
Growth of the U.S. IVF services market is driven by various factors, with one significant factor being the notable increase in infertility rates. Infertility affects a considerable number of couples in the U.S., with approximately one in eight couples experiencing difficulties in conceiving.
The demand for IVF treatments is expected to increase in Asia Pacific region owing to the growth in fertility tourism, increase in the number of international companies trying to penetrate economically developing countries, and change in the regulatory landscape in APAC. The Asia Pacific Initiative on Reproduction (ASPIRE) is a task force of clinicians & scientists engaged in the management of fertility & ART. This promotes awareness regarding infertility & ART and enhances infertility-related services in the region. According to OECD, in 2022, birth rate in Asia Pacific has fallen to population replacement rate of 2.1 born children per woman. Decrease in birth rate and increase in the geriatric population are among the key factors expected to propel market growth.
India IVF Market
India experienced a remarkable increase in the demand for IVF services. This surge can be attributed to several factors, including delayed marriages, a rise in the average age of pregnancy, an increase in infertility rates, higher disposable income levels, and awareness about the availability of infertility treatments. The average cost of IVF services in India range from USD 1,000 to USD 3,000.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the In vitro fertilization market
By Instrument
By Procedure Type
By Providers
By Regional
Chapter 1. Methodology and Scope
1.1. Market Segmentation
1.2. Market Definitions
1.2.1. Procedure Type
1.2.2. Providers
1.2.3. Instrument
1.2.4. Region
1.3. Estimates and Forecast Timeline
1.4. Research Methodology
1.4.1. Information procurement
1.4.2. Purchased Database
1.4.3. Internal Database
1.4.4. Secondary Sources
1.4.5. Primary Research
1.4.6. Details of Primary Research
1.5. Information or Data Analysis
1.5.1. Data Analysis Models
1.6. Market Formulation & Validation
1.6.1. Volume Price Analysis
1.6.2. Commodity Flow Analysis
1.7. List of Secondary Sources
1.8. List of Abbreviations
1.9. Research Objectives
Chapter 2. Executive Summary
2.1. Market Snapshot
2.2. Segment Snapshot
2.2.1. Procedure Type
2.2.2. End-Use
2.2.3. Instrument
2.3. Competitive Landscape Snapshot
Chapter 3. Market Variables, Trends, & Scope
3.1. Market Lineage Outlook
3.1.1. Parent market outlook
3.2. Market Segmentation
3.3. User Perspective Analysis
3.3.1. Consumer Behavior Analysis
3.3.2. Market Influencer Analysis
3.4. IVF Procedure Pricing Analysis
3.5. Market Dynamics
3.5.1. Market Driver Analysis
3.5.1.1. Rising reprotourism
3.5.1.2. Technological advancements in IVF
3.5.1.3. Favorable government funding
3.5.1.4. Late initiation of family
3.5.1.5. Increasing incidence rate of male and female infertility
3.5.1.6. Mergers and acquisitions (M&A)
3.5.2. Market Restraint Analysis
3.5.2.1. High cost of IVF treatment
3.5.2.2. Lack of regulatory framework and uniform regulations
3.5.2.3. Vulnerability of women depending on cross-border reprotourism
3.6. Industry Analysis Tools
3.6.1. Porter's Five Forces Analysis
3.6.2. PESTLE Analysis
3.6.3. Qualitative Analysis: Impact of COVID-19 on IVF Market
Chapter 4. In Vitro Fertilization Market: Procedure Type Analysis
4.1. In Vitro Fertilization by Procedure Type Market Share Analysis, 2023 & 2030
4.2. Segment Dashboard
4.3. Global In Vitro Fertilization Procedures Market, by Type, 2018 - 20302018 - 20302018 - 2030
4.4. Market Size and Forecasts and Trend Analysis, 2018 - 20302018 - 20302018 - 2030 for the Procedure Type
4.4.1. Fresh Nondonor
4.4.1.1. Fresh nondonor market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
4.4.2. Frozen Nondonor
4.4.2.1. Frozen nondonor market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
4.4.3. Fresh Donor
4.4.3.1. Fresh donor market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
4.4.4. Frozen Donor
4.4.4.1. Frozen donor market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
Chapter 5. In Vitro Fertilization Market: Providers Analysis
5.1. In Vitro Fertilization Market: Providers Market Share Analysis, 2023 & 2030
5.2. Segment Dashboard
5.3. Market Size and Forecasts and Trend Analysis, 2018 - 20302018 - 20302018 - 2030 for the following Providers
5.3.1. Fertility Clinics
5.3.1.1. Fertility clinics market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
5.3.2. Hospitals and Other Settings
5.3.2.1. Hospitals and other settings market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
Chapter 6. In Vitro Fertilization Market: Instrument Analysis
6.1. In Vitro Fertilization (IVF) Market Share Analysis, 2023 & 2030
6.2. Segment Dashboard
6.3. Global IVF Market, by Instrument, 2023 to 2030
6.4. Market Size & Forecasts and Trend Analysis, 2018 to 2030 for the Instrument
6.4.1. Culture Media
6.4.1.1. Culture media market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.1.2. Cryopreservation Media
6.4.1.2.1. Cryopreservation market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.1.3. Embryo Culture Media
6.4.1.3.1. Embryo culture media market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.1.4. Ovum Processing Media
6.4.1.4.1. Ovum processing media market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.1.5. Sperm Processing Media
6.4.1.5.1. Sperm processing media market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.2. Disposable Devices
6.4.2.1. Disposable devices market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3. Equipment
6.4.3.1. Equipment market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.2. Sperm Analyzer Systems Media
6.4.3.2.1. Sperm analyzer systems market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.3. Imaging Systems
6.4.3.3.1. Imaging systems market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.4. Ovum Aspiration Pumps
6.4.3.4.1. Ovum aspiration pumps market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.5. Micromanipulator Systems
6.4.3.5.1. Micromanipulator systems market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.6. Incubators
6.4.3.6.1. Incubators market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.7. Gas Analyzers
6.4.3.7.1. Gas analyzers market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.8. Laser Systems
6.4.3.8.1. Laser systems market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.9. Cryosystems
6.4.3.9.1. Cryosystems market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.10. Sperm Separation Devices
6.4.3.10.1. Sperm separation devices market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.11. IVF Cabinets
6.4.3.11.1. IVF cabinets market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.12. Anti-vibration Tables
6.4.3.12.1. Anti-vibration tables market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.13. Witness Systems
6.4.3.13.1. Witness systems market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
6.4.3.14. Others
6.4.3.14.1. Others market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
Chapter 7. Regional Outlook
7.1. IVF Market by Region: Key Marketplace Takeaway
7.2. North America
7.2.1. North America IVF market, 2018 - 2030 (USD Million)
7.2.2. U.S.
7.2.2.1. U.S. estimated fertility rate, 2018 - 2030 (%)
7.2.2.2. Regulatory framework
7.2.2.3. Reimbursement scenario
7.2.2.4. Competitive scenario
7.2.2.5. U.S. IVF market, 2018 - 2030 (USD Million)
7.2.3. Canada
7.2.3.1. Canada estimated fertility rate, 2018 - 2030 (%)
7.2.3.2. Regulatory framework
7.2.3.3. Reimbursement scenario
7.2.3.4. Competitive scenario
7.2.3.5. Canada IVF market, 2018 - 2030 (USD Million)
7.3. Europe
7.3.1. Europe IVF market, 2018 - 2030 (USD Million)
7.3.2. France
7.3.2.1. France estimated fertility rate, 2018 - 2030 (%)
7.3.2.2. Regulatory framework
7.3.2.3. Reimbursement scenario
7.3.2.4. Competitive scenario
7.3.2.5. France IVF market, 2018 - 2030 (USD Million)
7.3.3. Germany
7.3.3.1. Germany estimated fertility rate, 2018 - 2030 (%)
7.3.3.2. Regulatory framework
7.3.3.3. Reimbursement Scenario
7.3.3.4. Competitive scenario
7.3.3.5. Germany IVF market, 2018 - 2030 (USD Million)
7.3.4. Italy
7.3.4.1. Italy estimated fertility rate, 2018 - 2030 (%)
7.3.4.2. Regulatory framework
7.3.4.3. Reimbursement scenario
7.3.4.4. Competitive scenario
7.3.4.5. Italy IVF market, 2018 - 2030 (USD Million)
7.3.5. Spain
7.3.5.1. Spain estimated fertility rate, 2018 - 2030 (%)
7.3.5.2. Regulatory framework
7.3.5.3. Reimbursement scenario
7.3.5.4. Competitive scenario
7.3.5.5. Spain IVF market, 2018 - 2030 (USD Million)
7.3.6. UK
7.3.6.1. UK estimated fertility rate, 2018 - 2030 (%)
7.3.6.2. Regulatory framework
7.3.6.3. Reimbursement scenario
7.3.6.4. Competitive scenario
7.3.6.5. UK IVF market, 2018 - 2030 (USD Million)
7.3.7. Belgium
7.3.7.1. Belgium estimated fertility rate, 2018 - 2030 (%)
7.3.7.2. Regulatory framework
7.3.7.3. Reimbursement scenario
7.3.7.4. Competitive scenario
7.3.7.5. Belgium IVF market, 2018 - 2030 (USD Million)
7.3.8. Netherlands
7.3.8.1. Netherlands estimated fertility rate, 2018 - 2030 (%)
7.3.8.2. Regulatory framework
7.3.8.3. Reimbursement scenario
7.3.8.4. Competitive scenario
7.3.8.5. Netherlands IVF market, 2018 - 2030 (USD Million)
7.3.9. Switzerland
7.3.9.1. Switzerland estimated fertility rate, 2018 - 2030 (%)
7.3.9.2. Regulatory framework
7.3.9.3. Reimbursement scenario
7.3.9.4. Competitive scenario
7.3.9.5. Switzerland IVF market, 2018 - 2030 (USD Million)
7.3.10. Sweden
7.3.10.1. Sweden estimated fertility rate, 2018 - 2030 (%)
7.3.10.2. Regulatory framework
7.3.10.3. Reimbursement scenario
7.3.10.4. Competitive scenario
7.3.10.5. Sweden IVF market, 2018 - 2030 (USD Million)
7.3.11. Denmark
7.3.11.1. Denmark estimated fertility rate, 2018 - 2030 (%)
7.3.11.2. Regulatory framework
7.3.11.3. Reimbursement scenario
7.3.11.4. Competitive scenario
7.3.11.5. Denmark IVF market, 2018 - 2030 (USD Million)
7.3.12. Norway
7.3.12.1. Norway estimated fertility rate, 2018 - 2030 (%)
7.3.12.2. Regulatory framework
7.3.12.3. Reimbursement scenario
7.3.12.4. Competitive scenario
7.3.12.5. Norway IVF market, 2018 - 2030 (USD Million)
7.4. Asia Pacific
7.4.1. Asia Pacific IVF market, 2018 - 2030 (USD Million)
7.4.2. Japan
7.4.2.1. Japan estimated fertility rate, 2018 - 2030 (%)
7.4.2.2. Regulatory framework
7.4.2.3. Reimbursement scenario
7.4.2.4. Competitive scenario
7.4.2.5. Japan IVF market, 2018 - 2030 (USD Million)
7.4.3. China
7.4.3.1. China estimated fertility rate, 2018 - 2030 (%)
7.4.3.2. Regulatory framework
7.4.3.3. Reimbursement scenario
7.4.3.4. Competitive scenario
7.4.3.5. China IVF market, 2018 - 2030 (USD Million)
7.4.4. India
7.4.4.1. India estimated fertility rate, 2018 - 2030 (%)
7.4.4.2. Regulatory framework
7.4.4.3. Reimbursement scenario
7.4.4.4. Competitive scenario
7.4.4.5. India IVF market, 2018 - 2030 (USD Million)
7.4.5. Australia
7.4.5.1. Australia estimated fertility rate, 2018 - 2030 (%)
7.4.5.2. Regulatory framework
7.4.5.3. Reimbursement scenario
7.4.5.4. Competitive scenario
7.4.5.5. Australia IVF market, 2018 - 2030 (USD Million)
7.4.6. South Korea
7.4.6.1. South Korea estimated fertility rate, 2018 - 2030 (%)
7.4.6.2. Regulatory framework
7.4.6.3. Reimbursement scenario
7.4.6.4. Competitive scenario
7.4.6.5. South Korea IVF market, 2018 - 2030 (USD Million)
7.4.7. Thailand
7.4.7.1. Thailand estimated fertility rate, 2018 - 2030 (%)
7.4.7.2. Regulatory framework
7.4.7.3. Reimbursement scenario
7.4.7.4. Competitive scenario
7.4.7.5. Thailand IVF market, 2018 - 2030 (USD Million)
7.5. Latin America
7.5.1. Latin America IVF market, 2018 - 2030 (USD Million)
7.5.2. Brazil
7.5.2.1. Brazil estimated fertility rate, 2018 - 2030 (%)
7.5.2.2. Regulatory framework
7.5.2.3. Reimbursement scenario
7.5.2.4. Competitive scenario
7.5.2.5. Brazil IVF market, 2018 - 2030 (USD Million)
7.5.3. Mexico
7.5.3.1. Mexico estimated fertility rate, 2018 - 2030 (%)
7.5.3.2. Regulatory framework
7.5.3.3. Reimbursement scenario
7.5.3.4. Competitive scenario
7.5.3.5. Mexico IVF market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
7.5.4. Argentina
7.5.4.1. Argentina estimated fertility rate, 2018 - 2030 (%)
7.5.4.2. Regulatory framework
7.5.4.3. Reimbursement scenario
7.5.4.4. Competitive scenario
7.5.4.5. Argentina IVF market, 2018 - 20302018 - 20302018 - 2030 (USD Million)
7.6. Middle East & Africa
7.6.1. MEA IVF market, 2018 - 2030 (USD Million)
7.6.2. South Africa
7.6.2.1. South Africa estimated fertility rate, 2018 - 2030 (%)
7.6.2.2. Regulatory framework
7.6.2.3. Reimbursement scenario
7.6.2.4. Competitive scenario
7.6.2.5. South Africa IVF market, 2018 - 2030 (USD Million)
7.6.3. Saudi Arabia
7.6.3.1. Saudi Arabia estimated fertility rate, 2018 - 2030 (%)
7.6.3.2. Regulatory framework
7.6.3.3. Reimbursement scenario
7.6.3.4. Competitive scenario
7.6.3.5. Saudi Arabia IVF market, 2018 - 2030 (USD Million)
7.6.4. UAE
7.6.4.1. UAE estimated fertility rate, 2018 - 2030 (%)
7.6.4.2. Regulatory framework
7.6.4.3. Reimbursement scenario
7.6.4.4. Competitive scenario
7.6.4.5. UAE IVF market, 2018 - 2030(USD Million)
Chapter 8. Competitive Analysis
8.1. Company Profiles
8.1.1. Suppliers
8.1.1.1. Vitrolife
8.1.1.1.1. Overview
8.1.1.1.2. Financial Performance
8.1.1.1.3. Product Benchmarking
8.1.1.1.4. Strategic Initiatives
8.1.1.2. Emd Serono, Inc.
8.1.1.2.1. Overview
8.1.1.2.2. Financial Performance
8.1.1.2.3. Product Benchmarking
8.1.1.2.4. Strategic Initiatives
8.1.1.3. FUJIFILM Irvine Scientific
8.1.1.3.1. Overview
8.1.1.3.2. Financial Performance
8.1.1.3.3. Product Benchmarking
8.1.1.3.4. Strategic Initiatives
8.1.1.4. The Cooper Companies, Inc.
8.1.1.4.1. Overview
8.1.1.4.2. Financial Performance
8.1.1.4.3. Product Benchmarking
8.1.1.4.4. Strategic Initiatives
8.1.1.5. Thermo Fisher Scientific, Inc.
8.1.1.5.1. Overview
8.1.1.5.2. Financial Performance
8.1.1.5.3. Product Benchmarking
8.1.1.5.4. Strategic Initiatives
8.1.1.6. Bayer AG
8.1.1.6.1. Overview
8.1.1.6.2. Financial Performance
8.1.1.6.3. Product Benchmarking
8.1.1.6.4. Strategic Initiatives
8.1.1.7. Merck & Co., Inc.
8.1.1.7.1. Overview
8.1.1.7.2. Financial Performance
8.1.1.7.3. Product Benchmarking
8.1.1.7.4. Strategic Initiatives
8.1.1.8. Cook Medical LLC (Cook Group)
8.1.1.8.1. Overview
8.1.1.8.2. Financial Performance
8.1.1.8.3. Product Benchmarking
8.1.1.8.4. Strategic Initiatives
8.1.1.9. Genea Biomedx
8.1.1.9.1. Overview
8.1.1.9.2. Financial Performance
8.1.1.9.3. Product Benchmarking
8.1.1.9.4. Strategic Initiatives
8.1.1.10. Ferring Pharmaceutical B.V.
8.1.1.10.1. Overview
8.1.1.10.2. Financial Performance
8.1.1.10.3. Product Benchmarking
8.1.1.10.4. Strategic Initiatives
8.1.2. Service Providers
8.1.2.1. Nova IVF
8.1.2.1.1. Overview
8.1.2.1.2. Financial Performance
8.1.2.1.3. Service Benchmarking
8.1.2.1.4. Strategic Initiatives
8.1.2.2. Boston IVF
8.1.2.2.1. Overview
8.1.2.2.2. Financial Performance
8.1.2.2.3. Service Benchmarking
8.1.2.2.4. Strategic Initiatives
8.1.2.3. RMA Network (Reproductive Medicine Associates)
8.1.2.3.1. Overview
8.1.2.3.2. Financial Performance
8.1.2.3.3. Service Benchmarking
8.1.2.3.4. Strategic Initiatives
8.1.2.4. TFP Thames Valley Fertility
8.1.2.4.1. Overview
8.1.2.4.2. Financial Performance
8.1.2.4.3. Service Benchmarking
8.1.2.4.4. Strategic Initiatives
8.1.2.5. Fortis Healthcare
8.1.2.5.1. Overview
8.1.2.5.2. Financial Performance
8.1.2.5.3. Service Benchmarking
8.1.2.5.4. Strategic Initiatives
8.1.2.6. U.S. Fertility
8.1.2.6.1. Overview
8.1.2.6.2. Financial Performance
8.1.2.6.3. Service Benchmarking
8.1.2.6.4. Strategic Initiatives
8.2. Company Categorization
8.3. Company Market Position Analysis
8.4. Strategy Mapping
Chapter 9. Recommendations/Key Market Insights