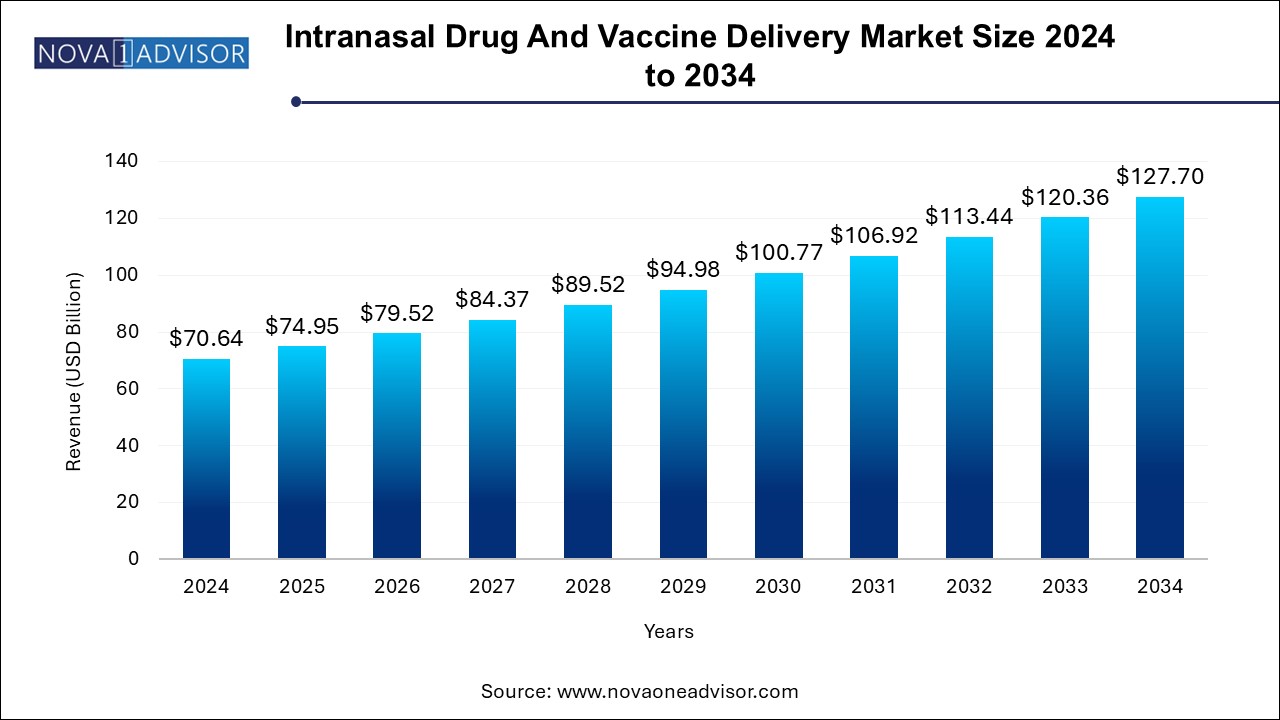
The intranasal drug and vaccine delivery market size was exhibited at USD 70.64 billion in 2024 and is projected to hit around USD 127.70 billion by 2034, growing at a CAGR of 6.1% during the forecast period 2024 to 2034. The growing demand for non-invasive drug delivery methods and the need for efficient vaccine administration are driving the intranasal drug and vaccine delivery market.
The intranasal drug and vaccine delivery market is gaining significant momentum due to its non-invasive nature, enhanced bioavailability, and ease of administration. By delivering drugs and vaccines directly through the nasal cavity, this method bypasses the digestive system, offering faster absorption and quicker onset of action. The rising prevalence of chronic diseases, respiratory disorders, and neurological conditions is boosting the demand for intranasal treatments. The market is characterized by a diverse range of products, including powder delivery devices, metered spray pumps, and pressurized metered-dose inhalers.
| Report Coverage | Details |
| Market Size in 2025 | USD 74.95 Billion |
| Market Size by 2034 | USD 127.70 Billion |
| Growth Rate From 2024 to 2034 | CAGR of 6.1% |
| Base Year | 2024 |
| Forecast Period | 2024-2034 |
| Segments Covered | Product, Dosage, Application, Distribution Channel, Region |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Key Companies Profiled | GSK plc; Teva Pharmaceutical Industries Ltd; AptarGroup, Inc.; UCB S.A., Belgium; Teleflex Incorporated; 3M; Bespak Limited; Optinose; Intersect ENT |
Driver: Growing Demand for Non-Invasive Drug Delivery
The increasing preference for non-invasive drug delivery methods is a major driver of the intranasal drug delivery market. With patients seeking less painful and more convenient alternatives to traditional injections, intranasal delivery offers a compelling solution. Intranasal drugs, particularly those for respiratory and neurological conditions, are quickly absorbed into the bloodstream through the nasal mucosa. This method also reduces the need for medical supervision, allowing for better patient compliance and reducing healthcare costs. Moreover, intranasal drug delivery bypasses the digestive system, increasing bioavailability and improving the overall effectiveness of medications.
Restraint: Limited Awareness and Regulatory Challenges
Despite the growing interest in intranasal drug delivery, limited awareness and regulatory hurdles pose challenges. Many patients and healthcare providers remain unfamiliar with the advantages of intranasal treatments, limiting market adoption. Furthermore, regulatory bodies often require extensive clinical trials to ensure the safety and efficacy of intranasal drug and vaccine formulations. These regulatory challenges, along with the need for specialized manufacturing processes, can slow down the market growth and make it more difficult for smaller companies to enter the market.
Opportunity: Advancements in Vaccine Delivery
Advancements in intranasal vaccine delivery present significant opportunities for growth. The intranasal route is gaining attention as a potential alternative to traditional injection-based vaccines, offering benefits such as ease of administration, pain-free delivery, and enhanced patient compliance. The success of intranasal vaccines has spurred investment and research into new vaccine formulations that can be delivered through the nasal cavity. As the technology matures, it could revolutionize vaccination programs, especially in underserved regions, providing a faster and more cost-effective means of delivering vaccines to large populations.
Liquid delivery devices dominated the market with a revenue share of 43.7% in 2024. These devices are highly valued due to their ability to bypass first-pass metabolism, improving bioavailability and enhancing the therapeutic effects of the delivered drugs. They are also non-invasive, which significantly boosts patient compliance, making them a preferred choice for chronic conditions requiring regular medication. Furthermore, recent technological advancements have led to the development of more user-friendly devices that support self-administration, further promoting their adoption in both clinical and home settings.
The Powder delivery devices, however, are projected to grow rapidly in the coming years. These devices provide accurate dosing and optimize absorption via the nasal mucosa. Their user-friendly designs and the latest innovations in compressed gas systems have contributed to their growing popularity, allowing for targeted delivery that improves therapeutic outcomes.
Multi-dose systems led the market with a revenue share of 64.1% in 2024. driven by their convenience and cost-effectiveness. Multi-dose systems are particularly beneficial for patients with chronic conditions as they reduce the need for frequent refills, improving patient adherence to treatment regimens. Their non-invasive nature further promotes compliance, making them highly preferred in healthcare settings.
On the other hand, the unit-dose segment is expected to witness the fastest growth, fueled by the increasing demand for single-use formulations, especially in vaccination. Unit-dose systems provide enhanced safety and efficacy, simplifying the administration process and improving patient compliance. The increasing approval of nasal sprays, such as naloxone, has amplified the popularity of unit-dose products.
Respiratory disorders held the largest market share of 41.1% in 2024, driven by the growing adoption of intranasal drug delivery systems for conditions such as asthma, chronic obstructive pulmonary disease (COPD), and allergic rhinitis. These devices offer rapid action and increased bioavailability, making them a preferred treatment option for patients with respiratory conditions. The non-invasive nature of intranasal delivery also enhances patient compliance, particularly for individuals who are averse to injectable treatments.
The vaccination segment is expected to register the fastest CAGR of 8.1% over the forecast period. Intranasal vaccines, particularly those administered without needles, are increasingly popular due to their ease of use and improved patient compliance, especially among children and needle-phobic individuals. The success of intranasal has accelerated the adoption of this delivery method, which provides localized and systemic immunity, making it highly effective for mass vaccination efforts.
Retail pharmacies dominated the market and accounted for a share of 46.0% in 2024, driven by the growing trend of self-administration and rising awareness among patients. Retail pharmacies offer a broad selection of intranasal products, making them highly accessible to consumers. Additionally, collaborations between device manufacturers and pharmacy chains have made it easier for patients to access point-of-care medications without the need for a prescription.
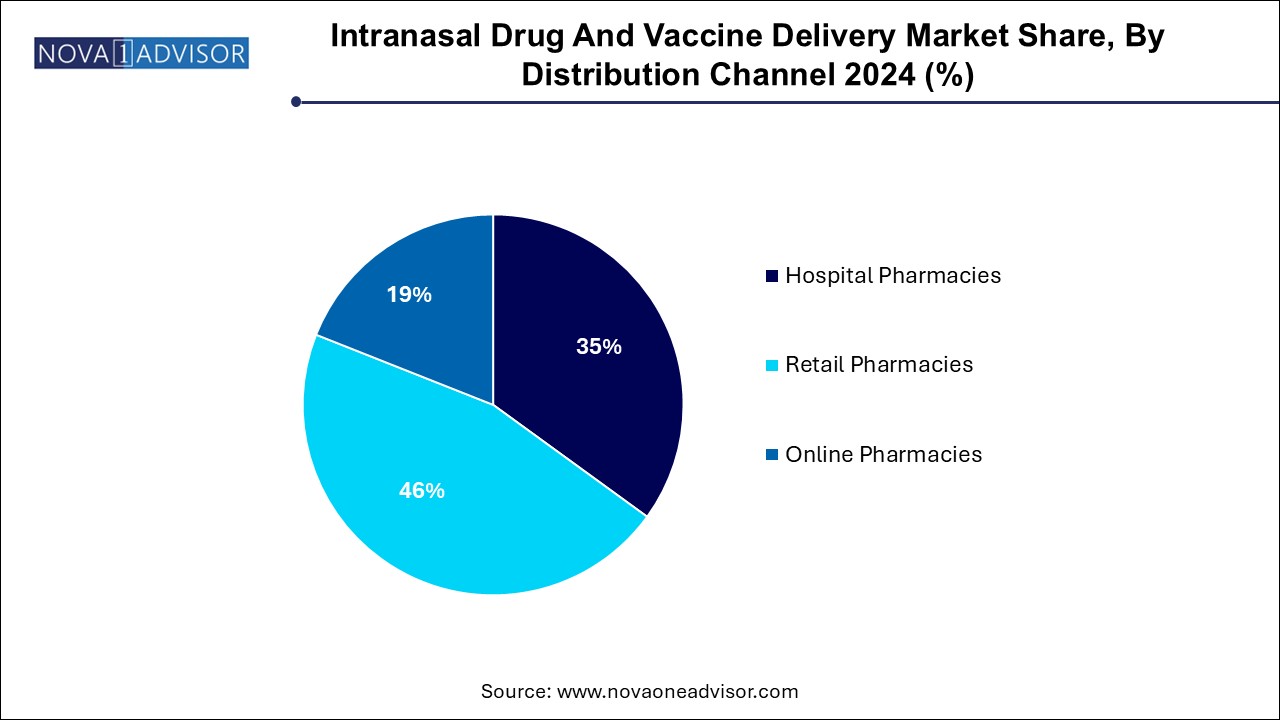
Online pharmacies are projected to grow at the fastest CAGR of 7.8% over the forecast period. The convenience of ordering intranasal medications from home, along with competitive pricing and discounts, has contributed to the increasing preference for online platforms. The rise of telemedicine and increased awareness of intranasal therapies further boost the demand for online pharmacies, making them a key distribution channel in the healthcare landscape.
North America holds the largest share of the intranasal drug and vaccine delivery market, driven by the region’s advanced healthcare infrastructure, high healthcare expenditure, and early adoption of new technologies. The U.S. is a major contributor to this dominance, with several leading pharmaceutical companies focusing on developing intranasal vaccines and treatments. Furthermore, the presence of regulatory agencies like the FDA facilitates the approval of new intranasal drug delivery systems, further driving the market in the region.
Asia-Pacific is expected to experience the fastest growth in the intranasal drug delivery market. This growth can be attributed to the increasing prevalence of respiratory disorders, rising healthcare awareness, and the growing demand for cost-effective treatments. Additionally, the expanding pharmaceutical and biotechnology industries in countries like India and China, along with supportive government initiatives, make the Asia-Pacific region an emerging market for intranasal drug and vaccine delivery systems.
In January 2025, a leading pharmaceutical company, XYZ Pharma, announced the successful completion of Phase 3 trials for its intranasal flu vaccine, which is set to revolutionize the vaccination process.
In October 2024, ABC Health, a startup specializing in intranasal drug delivery systems, secured a partnership with a major medical device manufacturer to enhance the distribution of its novel intranasal pain management device across Europe.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the intranasal drug and vaccine delivery market
Product
Dosage
Application
Distribution Channel
Regional