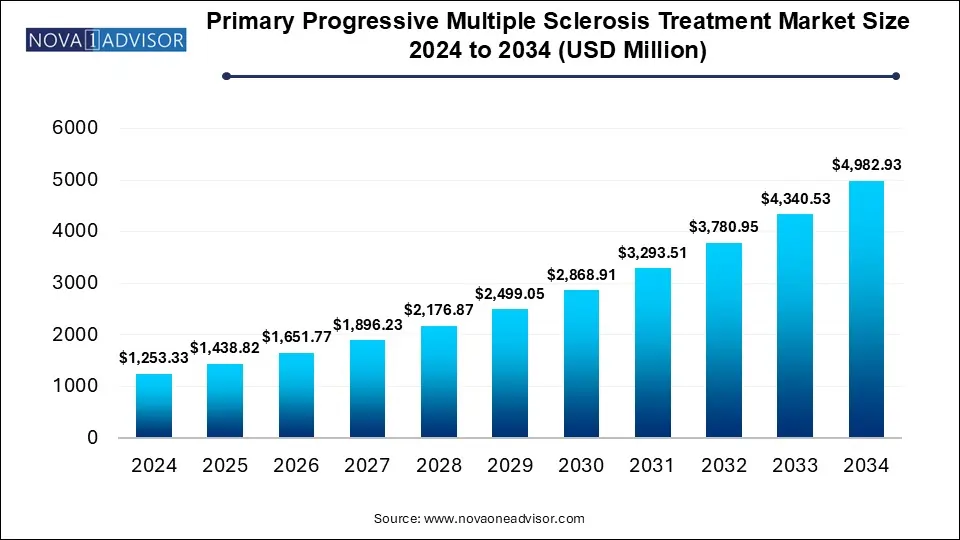
The global primary progressive multiple sclerosis treatment market size was reached at USD 1253.33 million in 2024 and is expected to hit around USD 4982.93 million by 2034, exhibiting a compound annual growth rate (CAGR) of 14.8% during the forecast period 2025 to 2034. The growing research and developments for personalized medicines are driving the primary progressive multiple sclerosis treatment market. Government initiatives and funding for novel treatments fueling the market.
The primary progressive multiple sclerosis treatment market is witnessing significant growth due to factors like increased prevalence of disease, ongoing research and development activities, advancements in diagnostic techniques like MRI and genetic therapies, and government investments and funding for disease-related research and development. Advancements in personalized medicines, disease-modifying therapies, and symptomatic treatments are leveraging favorable approaches in the treatment areas. Research institutes, pharmaceutical & biotechnology companies, and universities are raising their collaborative approaches to the development of novel therapies. Regulatory approvals for new drug developments and granting for clinical trials are contributing to further market growth.
The increased prevalence of primary progressive multiple sclerosis is a significant driver of the market. The prevalence of primary progressive multiple sclerosis has increased among the aging population. Environmental factors like vitamin D deficiency and smoking further contribute to the prevalence increasing. Advancement in scene diagnostic techniques is enabling early detection and accurate diagnosis of primary progressive multiple sclerosis. Increase prevalence, leading to rising awareness and driving demand for disease-modifying therapies.
Increased R&D Activities Poisoning Market Growth
The increasing R&D activities are holding significant growth opportunities for the primary progressive multiple sclerosis treatment market. Research and developments in stem cell therapies, gene therapies, immunomodulatory therapies, neuroprotective therapies, and biomarker-based treatments, enable more effective treatment solutions for disease. R&D activities are expanding the development of more effective and targeted treatments. The increased need for personalized medicine approaches is contributing to increased R&D activities. Additionally, government initiatives and funding for research and development are committed to developing innovative solutions for the disease. Regulatory approvals for novel treatment approaches are encouraging these activities.
Disease Management Complexity Hampering Market Growth
Primary progressive multiple sclerosis disease is heterogeneous and causes challenges in disease prediction and treatment response. The disease requires comprehensive management due to its multifaceted symptoms like motor, cognitive, and visual impairments. The lack of understanding of disease pathophysiology challenges the development of effective treatments and therapies. Additionally, high development costs for treatments become barriers to market growth.
| Report Coverage | Details |
| Market Size in 2025 | USD 1438.82 Million |
| Market Size by 2034 | USD 4982.93 Million |
| Growth Rate From 2025 to 2034 | CAGR of 14.8% |
| Base Year | 2024 |
| Forecast Period | 2025-2034 |
| Segments Covered | Drug Type, Distribution Channel, Region |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope | North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled | MedDay Pharma, Roche, MediciNova, Mapi Pharma, Brainstorm-Cell Therapeutics , Takeda Pharmaceuticals, International, Inc. , AB Science , Mallinckrodt , Atara Biotherapeutics, F. Hoffmann-La Roche Ltd. |
The approved drugs segment leads the market with the highest share due to regulatory approval for the sole medicine, Ocrevus, for primary progressive multiple sclerosis disease. Limited pipelines for drugs are driving the segment growth. Currently, Roche’s Ocrelizumab (Ocrevus) is the only approved treatment for the disease.
Pipeline drugs is the second largest segment leading the market. the increased research & development activities and government support contributing to rising pipelines for disease treatment solutions. Growing need for personalized medicines driving focus toward various mechanisms of action. Currently, 28 drugs are developed by companies and 2 by universities/institutes for primary progressive multiple sclerosis disease.
The Retail Pharmacies segment dominated the market in 2024. Retail pharmacies are convenient and accessible. The rising availability of medications in retail pharmacies is driving the segment growth. Favorable health reimbursement policies for multiple sclerosis at retail pharmacies are contributing to the segment growth.
However, the e-commerce segment is expected to lead the market in the forecast period. The wide reach and availability of medication on online platforms are driving the popularity of the e-commerce segment. E-commerce platforms are convenient and accessible. The e-commerce platform provides cost-effective options for patients, increasing medication purchases in online mode.
High Prevalence of Disease Dominating North American Market
North America is leading the global market due to advanced healthcare infrastructure and the presence of key market vendors. North America has a high prevalence of primary progressive multiple sclerosis disease. The region has a high adoption rate of disease-modifying therapies. North America has witnessed significant clinical trial approaches for immunostimulant therapies. Robust research institutes and collaborative approaches among universities and pharmaceutical companies are fueling the innovation and developments in primary progressive multiple sclerosis treatments.
The United States leads the regional market with a well-established healthcare infrastructure. The United States is facing a high prevalence of primary progressive multiple sclerosis disease. high levels of research and development, leading to innovative treatments and diagnostics. The regulatory approvals for novel drugs and treatment solutions play a vital role in market growth.
In September 2024, the FDA approved Genentech’s Ocrevus Zunovo, a subcutaneous formulation of the anti-CD20 antibody ocrelizumab, for the treatment of relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS). The formulation is offering a twice-a-year, healthcare professional-administered injection.
Asia Pacific Primary Progressive Multiple Sclerosis Treatment Market Trends
Asia Pacific is expected to witness significant market growth in the forecast period due to the large patient population, high investments in healthcare infrastructure, and growing awareness of treatment options. The awareness of primary progressive multiple sclerosis disease has increased in Asia. Available treatments and favorable policies are filling the market. government initiatives supporting healthcare and pharmaceutical development, driving the market expansion.
China is leading the regional market, driven by high patient populations and expanding healthcare infrastructure. Robust pharmaceutical and biotechnology industries in China are committed to innovation and the development of novel tailored treatment solutions. Additionally, government investments and funding in research & development are playing a favorable role in market growth.
According to Hansen et al. (2024), 30–40% of Chinese patients with MS (pwMS) were adherent to disease-modifying treatment (DMT).
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the Primary Progressive Multiple Sclerosis Treatment Market
By Drug Type
By Distribution Channel
By Regional