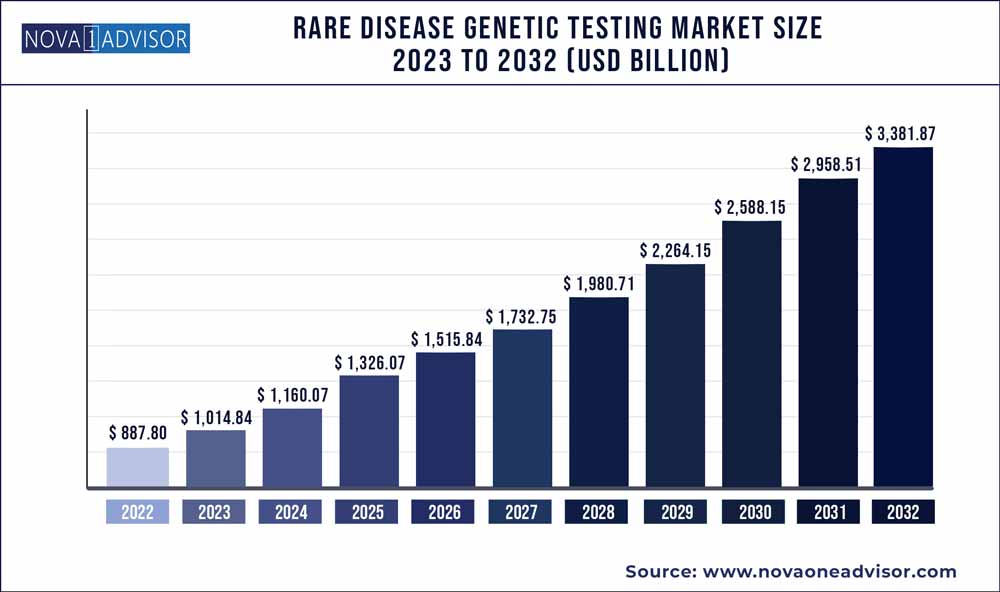
The global rare disease genetic testing market size was estimated at USD 887.80 million in 2022 and is expected to surpass around USD 3,381.87 million by 2032 and poised to grow at a compound annual growth rate (CAGR) of 14.31% during the forecast period 2023 to 2032. Advancements in technologies, such as NGS and microarray, are major drivers of the industry. The decline in sequencing prices has driven attention to the testing of rare diseases. In addition, the rising demand for early & rapid diagnosis is expected to foster the growth of the market. Furthermore, the critical role of translational research & genomic technologies to enhance the analysis and identification of novel mutations is expected to contribute to the industry growth. With more translational research activities in this area, the implementation of genetic testing in disease diagnostics is anticipated to witness high growth during the forecast period.
Key Takeaways:
Rare Disease Genetic Testing Market Report Scope
| Report Coverage | Details |
| Market Size in 2023 | USD 887.80 Million |
| Market Size by 2032 | USD 3,381.87 Million |
| Growth Rate From 2023 to 2032 | CAGR of 14.31% |
| Base Year | 2022 |
| Forecast Period | 2023-2032 |
| Segments Covered | Disease Type, Specialty, Technology, End-use, Region |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Regional Scope | North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa |
| Key Companies Profiled | Quest Diagnostics, Inc.; Centogene N.V.; Invitae Corp.; 3billion, Inc.; Arup Laboratories; Eurofins Scientific; Strand Life Sciences; Ambry Genetics; Perkin Elmer, Inc.; Macrogen, Inc.; Baylor Genetics; Color Genomics, Inc.; Health Network Laboratories; PreventionGenetics; Progenity, Inc.; Coopersurgical, Inc.; Fulgent Genetics Inc.; Myriad Genetics, Inc.; Laboratory Corporation Of America Holdings; Opko Health, Inc.; Artemis DNA |
Amidst the COVID-19 pandemic, patients with undiagnosed and rare diseases have been facing significant health challenges. According to a study published in January 2021 by a group of researchers from the U.S., there is an urgent need for the development of approaches that can reduce the serious challenges affecting rare and undiagnosed disease patients and families. The challenges include diagnostic and/or prognostic uncertainty coupled with medical complexity leading to poor health outcomes. In addition, clinical trials focused on rare diseases have also been affected by the COVID-19 pandemic. The pandemic has created several challenges for clinical trials. The challenges associated with finding, recruiting, and retaining patients with rare diseases have majorly impacted clinical trials.
Establishing a specific diagnosis for rare diseases is one of the primary challenges for clinicians and thus incurs a significant healthcare expenditure worldwide. However, the advent of NGS technologies has significantly driven research and diagnosis in this area. NGS has not only improved the diagnostic workflows but has also played a vital role in providing an unprecedented procedure for deciphering novel disease-associated genes. Along with offering inexpensive and rapid genetic diagnosis, NGS has also highlighted the relevance of de novo and mosaic mutations, deciphered the wide phenotypic spectrum of genes, and identified the presence of more than one rare disease in the same patient or digenic inheritance. Furthermore, the plummeting cost of sequencing has driven the adoption of NGS specialty in disease management.
An increase in the number of available registries in this area is one of the major driving factors of the industry as it enables pool data to achieve a sufficient sample size for epidemiological and/or clinical research. They play a pivotal role in assessing the feasibility of clinical trials and thus facilitate the effective planning of clinical trials and enrolment of patients. Registries accelerate service planning as well as support public health and clinical research by delivering key insights to researchers. Furthermore, registries can be deployed for the creation and dissemination of new data findings to inform clinical best practice and care, along with enabling seamless incorporation of patient data in diagnostic procedures.
Ongoing scientific advances have created new opportunities for the development of solutions that can impact the management of the disease. Collaborations are being undertaken not only across diverse medical and scientific domains but also with various stakeholder groups, such as researchers, patients, and regulators, for rare disease product development. For instance, in June 2022, the U.S. FDA revealed its program for Rare Neurodegenerative Diseases, a 5-year strategy for extending and refining the lives of people suffering from rare diseases, by evolving the progress of effective & safe medical products and enabling patient access to innovative treatments.
In addition, strategic initiatives for new product development in rare disease diagnoses further drive the growth. For instance, in Feb 2022 Bionano Genomics launched its Rare Undiagnosed Genetic Disease (RUGD) initiative to help elevate the level of dedication and focus in clinical and translational research. Rare Undiagnosed Genetic Disease initiative will incorporate Bionano’s products for the diagnosis, advancement of educational awareness, working towards the expansion of research grants in rare diseases, and facilitating expert societies with a mission of refining rare undiagnosed genetic disease patient care and supervision, such as the American College of Medical Genetics and Genomics.
Disease Type Insights
The endocrine & metabolism diseases segment is expected to register the fastest growth rate of more than 21.0% during the forecast period. In recent years, the understanding of molecular and genetic causes of endocrine diseases, such as Cushing’s syndrome, has increased considerably. This boosts the adoption of genetic testing for endocrine diseases. Furthermore, the identification of inherited mutations in patients with the primary pigmented nodular adrenocortical disease and bilateral macronodular adrenal hyperplasia is anticipated to accelerate advancements in tools for genetic testing for early disease detection. The immunological disorders segment accounted for the second-highest revenue share.
Immunologic disorders, such as Multiple Sclerosis (MS), are among the most prevalent rare diseases. The genetic profile of MS is one of the key focus areas among researchers operating in this area. This is primarily to obtain relevant insights into the causes and underlying physiology of diseases. Furthermore, organizations, such as the Australian and New Zealand MS Genetics Consortium (ANZGene) that bring together molecular biologists, neurologists, geneticists, and bioinformaticians operating in this space to collaborate on research projects are anticipated to positively influence the segment growth.
Technology Insights
The Next-Generation Sequencing (NGS) technology segment dominated the global industry in 2022 and accounted for the maximum share of more than 35.22% of the overall revenue. and accounted for the maximum share of more than 35.40% of the overall revenue. Wide availability and adoption of NGS-based gene panels for cancer, neurologic disease, cardiovascular disease, pediatric conditions, psychiatric disorders, and other related disease testing have driven the segment. Strategic activities by key players are estimated to further drive the segment over the forecast period. For instance, in June 2022, Avesthagen Ltd. formed a strategic alliance with Wipro Ltd. for the commercialization of its genetic testing offerings.
The portfolio comprises genome panels providing highly précised, disease-centric analysis for diseases including autoimmune disorders, neurodegenerative diseases cancers, and rare diseases.WES is considered a high-potential genetic testing method in the case where the genetic cause of a rare disease is unknown and difficult to identify. WES is becoming the standard of care for patients with undiagnosed rare diseases. Exons make up around 1.5% of an individual’s genome and contain 85% of all known disease-causing mutations. Thus, WES plays a crucial role in obtaining insights into protein making and disease physiology.
Specialty Insights
Based on specialties, the global industry has been further categorized into molecular genetic tests, chromosomal genetic tests, and biochemical genetic tests. The molecular genetic tests specialty segment dominated the global industry in 2022 and accounted for the highest share of more than 41.10% of the global revenue. The segment will retain its dominant position growing at the fastest CAGR during the forecast period. Rapid technological advancements and expertise in handling & managing high throughput technologies within clinical settings are factors expected to boost the segment growth.
Molecular genetic tests enable investigating single genes or short lengths of DNA for the detection of mutations or variations leading to genetic disorders. Molecular testing is not only limited to rare diseases but also covers testing of ultra-rare diseases. Genome sequencing is the most advanced unbiased testing method and is readily available for both research and clinical settings. This is primarily on account of the declining costs of sequencing tests along with the continuous development of next-generation sequencing-based new tests.
End-use Insights
The research laboratories & CROs segment led the global industry in 2022 and accounted for the highest share of more than 46.93% of the overall revenue. Laboratories are the key end-users; in the majority of cases, blood samples collected from patients are sent to a laboratory for testing. Laboratories offer testing based on various specialties, including molecular genetic tests, chromosomal genetic tests, and biochemical genetic tests. Moreover, molecular genetic testing-based laboratory testing is rapidly increasing worldwide. Genetic tests are conducted by multiple laboratories, including those that are accredited with CLIA for clinical cytogenetics, pathology, and chemistry among other specialties.
The diagnostic laboratories segment is expected to register the fastest CAGR of 16.1% over the study period. The reason for its growth is the rising number of partnership and collaboration activities of diagnostic laboratories with genetic testing companies. In November 2021, Genomenon and Alexion, AstraZeneca Rare Disease announced a strategic collaboration projected to make important information for the treatment and diagnosis of rare diseases more readily available. The goal of the collaboration is to empower the genetic testing laboratories with the data they need for the diagnosis of rare diseases.
Regional Insights
Based on geographies, the industry has been further categorized into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America dominated the global industry with a share of more than 47.2% in 2022. The major share of the region can be attributed to the high incidence of rare diseases, a large number of disease registries, the presence of a substantial number of R&D facilities for ultra-rare diseases, and extensive investments in the diagnosis of disease. On the other hand, Asia Pacific is estimated to be the fastest-growing region at a CAGR of 18.1% during the forecast years.
This is mainly owing to the increase in awareness and diagnosis abilities. Furthermore, the introduction of policies and frameworks to promote disease management will offer lucrative opportunities in this region. For instance, in India, in March 2021, an inclusive National Policy for Rare Diseases was permitted by the Ministry of Health & Family Welfare. In addition, in 2017, a hospital-based National Registry for Rare Diseases was originated by the Indian Council of Medical Research by involving the centers across the country which are involved in the management and diagnosis of rare diseases.
Some of the prominent players in the Rare disease genetic testing Market include:
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2020 to 2032. For this study, Nova one advisor, Inc. has segmented the global Rare disease genetic testing market.
Disease Type
Technology
Specialty
End-Use
By Region