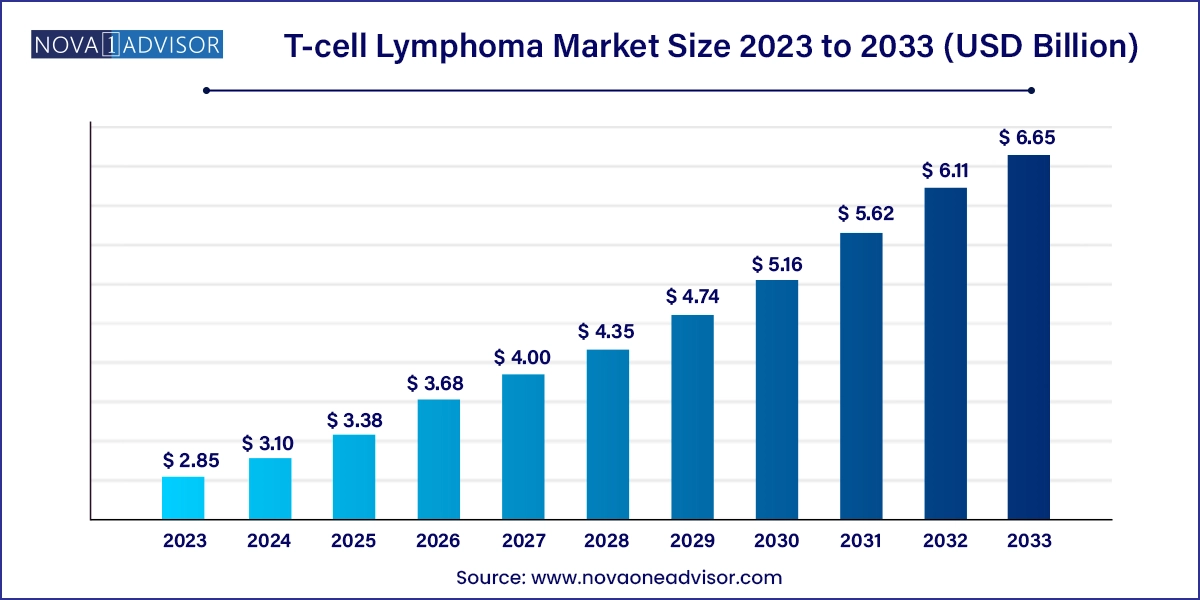
The global T-cell lymphoma market size was valued at USD 2.85 billion in 2023 and is anticipated to reach around USD 6.65 billion by 2033, growing at a CAGR of 8.85% from 2024 to 2033.
The T-cell Lymphoma (TCL) market is experiencing a period of substantial growth, driven by advancements in treatment options, and strategic initiatives by pharmaceutical companies. One of the primary factors for this growth is the introduction of new key pipeline agents and the expansion of existing therapies to new indications. These developments provide more options for patients and reduce the reliance on traditional chemotherapy regimens, which have been the mainstay of TCL treatment.
In 2023, there were approximately 12,600 new cases of Peripheral T-cell Lymphoma (PTCL) in the U.S. This number is expected to increase by 2030, reflecting a rising incidence of the disease. Additionally, the total incident cases of selected indications for CAR-T therapies in Non-Hodgkin Lymphoma (NHL) in the 7MM (the United States, France, Germany, Italy, Spain, UK, and Japan) comprised approximately 155,300 in 2023. The emerging pipeline of CAR-T therapies includes drugs at various stages of development-late-stage, mid-stage, and early-stage-targeting different lines of therapies and indications, mainly B-cell Lymphoma such as DLBCL, FL, MCL, MZL, and CLL/SLL, with one being developed for PTCL.
Currently, newly diagnosed PTCL patients are typically treated with anthracycline-based chemotherapy regimens. The standard initial treatment involves combination chemotherapy regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone). However, new treatment paradigms are emerging. For instance, CHP-BV (cyclophosphamide, doxorubicin, prednisone, and brentuximab vedotin) is a new regimen for ALCL, representing a shift towards more targeted therapies.
The introduction of new therapies such as HIYASTA for ATLL and PTCL, DARVIAS, and REMITORO for relapsed or refractory (R/R) ATLL and R/R PTCL in Japan provides additional treatment options and drives space growth. These new drugs offer hope for improved outcomes for patients who have limited treatment options. The field of CAR-T and NK (natural killer) cell treatments is also making inroads into TCL. Although these therapies are still in the preclinical phase, larger clinical studies are recruiting or underway. These innovative treatments hold promise for more effective and personalized approaches to TCL.
In addition to CAR-T and NK cell therapies, new biologics are being developed to target specific proteins and signals involved in TCL. These include drugs targeting programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1), NK-cell engagers targeting CD30/16a, the inhibition of 'Do Not Eat Me' signals by the anti-CD47 monoclonal antibody, and antibodies like mogamulizumab targeting CCR4. These advancements offer new opportunities to create highly disease-specific treatment platforms, potentially leading to better patient outcomes.
Several industry-changing events are anticipated between 2020 and 2030, particularly in Japan and the U.S. These events are expected to boost the total space size of TCL by 2030. Pharmaceutical companies are employing strategies such as introducing same-in-class drugs into new spaces where the mechanism of action (MOA) was previously unavailable, testing novel or existing MOAs in a TCL subtype-specific fashion, and focusing on new or under-researched targets.
| Report Attribute | Details |
| Market Size in 2024 | USD 3.10 Billion |
| Market Size by 2033 | USD 6.65 Billion |
| Growth Rate From 2024 to 2033 | CAGR of 8.85% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Type, therapy, region |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Key Companies Profiled | Acrotech Biopharma; Affimed GmbH; Bristol Myers Squibb; Chipscreen Biosciences; Citius Pharma; Daiichi Sankyo Company; Limited; Eisai Co., Ltd.; Genor Biopharma Co. Ltd; Innate Pharma; Dizal Pharma. |
The peripheral type segment dominated the market with the largest revenue share of over 66.87% in 2023. PTCL includes various subtypes, such as Angioimmunoblastic T-cell Lymphoma (AITL) and Anaplastic Large Cell Lymphoma (ALCL). These subtypes are commonly treated with anthracycline-based chemotherapy regimens like CHOP and CHOEP. However, recent advancements have introduced new therapies such as CHP-BV for ALCL and other targeted treatments. The increasing incidence of PTCL, with approximately 12,600 new cases in the U.S. in 2023, and the introduction of innovative treatment options are key factors driving the growth of this segment. Additionally, the launch of new therapies in regions like Japan provides further momentum, making PTCL the largest and most dynamic segment in the TCL market.
The Lymphoblastic T-cell Lymphoma (LBL) segment is experiencing moderate growth. LBL is a more aggressive form of lymphoma that often affects younger patients and requires intensive treatment regimens. Traditional chemotherapy remains a primary treatment option; however, there is ongoing research and development aimed at finding more effective therapies with fewer side effects. While the growth in this segment is not as rapid as in the PTCL segment, advancements in targeted therapies and the introduction of new treatment protocols are contributing to steady growth. The focus on improving patient outcomes and the development of new drugs are expected to drive continued interest and investment in the LBL segment, supporting its moderate growth trajectory.
The chemotherapy segment dominated the market with the largest revenue share in 2023. For many years, chemotherapy has been the cornerstone of TCL treatment, with regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone) being commonly used. These regimens are widely recognized for their effectiveness in managing disease and are typically the first line of treatment for newly diagnosed patients. Despite the emergence of new therapies, chemotherapy continues to be the primary choice due to its established protocols and widespread availability. The enduring reliance on these treatments sustains the prominence of the chemotherapy segment.
The Immunotherapy segment is expected to grow at the fastest growth rate over the forecast period. This rapid growth is driven by significant advancements and the introduction of novel therapies that harness the body's immune system to target cancer cells more precisely. For instance, CAR-T cell therapy, which involves modifying a patient's T cells to attack cancer cells, has shown promising results in clinical trials and is expanding its indications. Additionally, new biologics targeting specific proteins and pathways involved in TCL, such as the anti-CD47 monoclonal antibody and mogamulizumab, are being developed and introduced to the space. In December 2023, the launch of HIYASTA in Japan marked a significant step forward in immunotherapy options for patients with TCL. The continued innovation and increasing approval of these targeted treatments are expected to drive substantial growth in the immunotherapy segment, making it the fastest-growing area in the TCL.
North America T-cell lymphoma market held the largest share of 38.11% in 2023, driven by advanced healthcare infrastructure, high healthcare expenditure, and significant ongoing research and development. In the United States, which accounted for the high number of incident cases of CTCL in the 7MM in 2023, males had a higher incidence of CTCL. The sapce is characterized by the availability of advanced treatments like VALCHLOR, POTELIGEO, and ADCETRIS. The prevalence of TCL in the U.S. is projected to increase by 2030, further solidifying North America's dominance in the TCL market.
.webp)
U.S. T-cell Lymphoma Market Trends
The T-cell lymphoma market in the U.S. is majorly driven by a high incidence of the disease. The incidence of CTCL was high among the 7MM in 2023, with males representing a larger proportion of the patient population. Advanced diagnostic and treatment facilities contribute to the significant industry size. Emerging therapies like SGX301 and I/ONTAK are expected to enhance the treatment landscape from 2024 to 2030, addressing unmet needs and offering new standard care options for patients.
Europe T-cell Lymphoma Market Trends
The T-cell lymphoma market in Europe is growing due to substantial contributions from countries like the UK, France, and Germany. The region benefits from well-established healthcare systems and access to cutting-edge treatments. However, the market size varies across countries, with Germany holding the largest share among the EU4 and the UK in 2023.
The UK T-cell lymphoma market is growing steadily, driven by increased awareness and early diagnosis of the disease. The UK accounted for ~1,407 cases of Stage IA CTCL in 2023, reflecting a strong healthcare system capable of managing early-stage diseases effectively. Emerging therapies and clinical trials are expected to further bolster market growth.
The T-cell lymphoma market in France contributes significantly to the European TCL market. With a focus on innovative treatments and comprehensive healthcare coverage, France is well-positioned to adopt new therapies as they become available. The country continues to invest in research to address the unmet needs in TCL treatment.
Germany T-cell lymphoma market is expected to grow over the forecast period. Germany had the largest market size among the EU4 and the UK in 2023. The country’s robust healthcare infrastructure and focus on research and development enable it to be a leader in the adoption of new treatments. Germany's growth is expected to continue with the introduction of emerging therapies.
Asia Pacific T-cell Lymphoma Market Trends
The T-cell lymphoma market in Asia Pacific is experiencing rapid growth driven by increasing incidence rates and improving healthcare infrastructure. Countries like India, China, and Japan are significant contributors to this growth.
India T-cell lymphoma market is growing due to increasing awareness and improving healthcare access. While traditional chemotherapy remains prevalent, there is a growing interest in innovative treatments as the healthcare system evolves.
The T-cell lymphoma market in China is witnessing a rise in TCL cases, with significant investments in healthcare infrastructure and research. The country is focusing on adopting advanced therapies and improving diagnostic capabilities to manage the increasing patient load effectively.
Japan T-cell lymphoma market has a well-established market with advanced treatment options available. The introduction of new therapies like HIYASTA in December 2023 marks a significant development, enhancing the treatment landscape for TCL patients and driving space growth.
Latin America T-cell Lymphoma Market Trends
The T-cell lymphoma market in Latin America is growing, with Brazil being a key player. The region is gradually adopting advanced therapies, although access to cutting-edge treatments varies.
Brazil T-cell lymphoma market is a significant market in the Latin America region, with an increasing incidence of TCL and improving healthcare infrastructure. The country is focusing on enhancing access to advanced treatments and diagnostics to better manage the disease.
Middle East & Africa T-cell Lymphoma Market Trends
The T-cell lymphoma market in the Middle East & Africa is an emerging market for TCL, with countries like Saudi Arabia investing in healthcare improvements. The region faces challenges related to access to advanced treatments, but ongoing healthcare reforms are promising.
Saudi Arabia T-cell lymphoma market is actively investing in its healthcare system to improve access to advanced TCL treatments. The country's efforts to enhance healthcare infrastructure and adopt new therapies are expected to drive market growth in the region.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the T-cell Lymphoma market.
By Type
By Therapy
By Region
Chapter 1. Methodology And Scope
1.1. Market Segmentation & Scope
1.2. Segment Definitions
1.2.1. Type
1.2.2. Therapy
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased database
1.4.2. nova one advisors internal database
1.4.3. Secondary sources
1.4.4. Primary research
1.4.5. Details of primary research
1.4.5.1. Data for primary interviews in North America
1.4.5.2. Data for primary interviews in Europe
1.4.5.3. Data for primary interviews in Asia Pacific
1.4.5.4. Data for primary interviews in Latin America
1.4.5.5. Data for primary interviews in MEA
1.5. Information or Data Analysis
1.5.1. Data analysis models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.2. Approach 1: Commodity flow approach
1.7.3. Volume price analysis (Model 2)
1.7.4. Approach 2: Volume price analysis
1.8. List of Secondary Sources
1.9. List of Primary Sources
1.10. Objectives
Chapter 2. Market Variables, Trends, & Scope
2.1. Market Segmentation and Scope
2.2. Market Lineage Outlook
2.2.1. Parent Market Outlook
2.2.2. Related/Ancillary Market Outlook
2.3. Market Trends and Outlook
2.4. Market Dynamics
2.4.1. Advances in Immunotherapy and Targeted Therapies
2.4.2. Increasing Prevalence of T-cell Lymphoma Globally
2.4.3. Regulatory Approvals and Expanded Indications for Novel Treatments
2.5. Market Restraint Analysis
2.5.1. High Cost of Innovative Therapies
2.5.2. Limited Awareness and Diagnosis in Developing Regions
2.6. Business Environment Analysis
2.6.1. SWOT Analysis; By Factor (Political & Legal, Economic and Technological)
2.6.2. Porter’s Five Forces Analysis
2.7. COVID-19 Impact Analysis
Chapter 3. T-cell Lymphoma Market: Type Business Analysis
3.1. Type Market Share, 2024 - 2033
3.2. Segment Dashboard
3.3. T-cell Lymphomas Market by Type Outlook
3.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
3.5. Peripheral
3.5.1. Market Estimates and Forecasts, 2021 - 2033
3.5.2. Cutaneous T-cell Lymphoma
3.5.2.1. Market Estimates and Forecasts, 2021 - 2033
3.5.3. Anaplastic Large Cell Lymphoma
3.5.3.1. Market Estimates and Forecasts, 2021 - 2033
3.5.4. Angio-immuno-blastic T-cell Lymphoma
3.5.4.1. Market Estimates and Forecasts, 2021 - 2033
3.5.5. Other
3.5.5.1. Market Estimates and Forecasts, 2021 - 2033
3.6. Lymphoblastic
3.6.1. Market Estimates and Forecasts, 2021 - 2033
Chapter 4. T-cell Lymphoma Market: Therapy Business Analysis
4.1. Therapy Market Share, 2024 - 2033
4.2. Segment Dashboard
4.3. T-cell Lymphomas Market by Therapy Outlook
4.4. Market Size & Forecasts and Trend Analyses, 2018 to 2030 for the following
4.5. Radiotherapy
4.5.1. Market Estimates and Forecasts, 2021 - 2033
4.6. Chemotherapy
4.6.1. Market Estimates and Forecasts, 2021 - 2033
4.7. Immunotherapy
4.7.1. Market Estimates and Forecasts, 2021 - 2033
4.8. Stem Cell Transplantation
4.8.1. Market Estimates and Forecasts, 2021 - 2033
4.9. Other
4.9.1. Market Estimates and Forecasts, 2021 - 2033
Chapter 5. T-cell Lymphoma Market: Regional Business Analysis
5.1. T-cell Lymphoma Market Share By Region, 2024 - 2033
5.2. North America
5.2.1. U.S.
5.2.1.1. Key Country Dynamics
5.2.1.2. Target Disease Prevalence
5.2.1.3. Competitive Scenario
5.2.1.4. Regulatory Framework
5.2.1.5. Reimbursement Scenario
5.2.1.6. U.S. T-cell Lymphoma Market, 2021 - 2033
5.2.2. Canada
5.2.2.1. Key Country Dynamics
5.2.2.2. Target Disease Prevalence
5.2.2.3. Competitive Scenario
5.2.2.4. Regulatory Framework
5.2.2.5. Reimbursement Scenario
5.2.2.6. Canada T-cell Lymphoma Market, 2021 - 2033
5.2.3. Mexico
5.2.3.1. Key Country Dynamics
5.2.3.2. Target Disease Prevalence
5.2.3.3. Competitive Scenario
5.2.3.4. Regulatory Framework
5.2.3.5. Reimbursement Scenario
5.2.3.6. Mexico T-cell Lymphoma Market, 2021 - 2033
5.3. Europe
5.3.1. Europe T-cell Lymphomas Market, 2021 - 2033
5.3.2. Germany
5.3.2.1. Key Country Dynamics
5.3.2.2. Target Disease Prevalence
5.3.2.3. Competitive Scenario
5.3.2.4. Regulatory Framework
5.3.2.5. Reimbursement Scenario
5.3.2.6. Germany T-cell Lymphoma Market, 2021 - 2033
5.3.3. UK
5.3.3.1. Key Country Dynamics
5.3.3.2. Target Disease Prevalence
5.3.3.3. Competitive Scenario
5.3.3.4. Regulatory Framework
5.3.3.5. Reimbursement Scenario
5.3.3.6. UK T-cell Lymphoma Market, 2021 - 2033
5.3.4. France
5.3.4.1. Key Country Dynamics
5.3.4.2. Target Disease Prevalence
5.3.4.3. Competitive Scenario
5.3.4.4. Regulatory Framework
5.3.4.5. Reimbursement Scenario
5.3.4.6. France T-cell Lymphoma Market, 2021 - 2033
5.3.5. Italy
5.3.5.1. Key Country Dynamics
5.3.5.2. Target Disease Prevalence
5.3.5.3. Competitive Scenario
5.3.5.4. Regulatory Framework
5.3.5.5. Reimbursement Scenario
5.3.5.6. Italy T-cell Lymphoma Market, 2021 - 2033
5.3.6. Spain
5.3.6.1. Key Country Dynamics
5.3.6.2. Target Disease Prevalence
5.3.6.3. Competitive Scenario
5.3.6.4. Regulatory Framework
5.3.6.5. Reimbursement Scenario
5.3.6.6. Spain T-cell Lymphoma Market, 2021 - 2033
5.3.7. Denmark
5.3.7.1. Key Country Dynamics
5.3.7.2. Target Disease Prevalence
5.3.7.3. Competitive Scenario
5.3.7.4. Regulatory Framework
5.3.7.5. Reimbursement Scenario
5.3.7.6. Denmark T-cell Lymphoma Market, 2021 - 2033
5.3.8. Sweden
5.3.8.1. Key Country Dynamics
5.3.8.2. Target Disease Prevalence
5.3.8.3. Competitive Scenario
5.3.8.4. Regulatory Framework
5.3.8.5. Reimbursement Scenario
5.3.8.6. Sweden T-cell Lymphoma Market, 2021 - 2033
5.3.9. Norway
5.3.9.1. Key Country Dynamics
5.3.9.2. Target Disease Prevalence
5.3.9.3. Competitive Scenario
5.3.9.4. Regulatory Framework
5.3.9.5. Reimbursement Scenario
5.3.9.6. Norway T-cell Lymphoma Market, 2021 - 2033
5.4. Asia Pacific
5.4.1. Asia Pacific T-cell Lymphoma Market,, 2021 - 2033
5.4.2. Japan
5.4.2.1. Key Country Dynamics
5.4.2.2. Target Disease Prevalence
5.4.2.3. Competitive Scenario
5.4.2.4. Regulatory Framework
5.4.2.5. Reimbursement Scenario
5.4.2.6. Japan T-cell Lymphoma Market, 2021 - 2033
5.4.3. China
5.4.3.1. Key Country Dynamics
5.4.3.2. Target Disease Prevalence
5.4.3.3. Competitive Scenario
5.4.3.4. Regulatory Framework
5.4.3.5. Reimbursement Scenario
5.4.3.6. China T-cell Lymphoma Market, 2021 - 2033
5.4.4. India
5.4.4.1. Key Country Dynamics
5.4.4.2. Target Disease Prevalence
5.4.4.3. Competitive Scenario
5.4.4.4. Regulatory Framework
5.4.4.5. Reimbursement Scenario
5.4.4.6. India T-cell Lymphoma Market, 2021 - 2033
5.4.5. South Korea
5.4.5.1. Key Country Dynamics
5.4.5.2. Target Disease Prevalence
5.4.5.3. Competitive Scenario
5.4.5.4. Regulatory Framework
5.4.5.5. Reimbursement Scenario
5.4.5.6. South Korea T-cell Lymphoma Market, 2021 - 2033
5.4.6. Australia
5.4.6.1. Key Country Dynamics
5.4.6.2. Target Disease Prevalence
5.4.6.3. Competitive Scenario
5.4.6.4. Regulatory Framework
5.4.6.5. Reimbursement Scenario
5.4.6.6. Australia T-cell Lymphoma Market, 2021 - 2033
5.4.7. Thailand
5.4.7.1. Key Country Dynamics
5.4.7.2. Target Disease Prevalence
5.4.7.3. Competitive Scenario
5.4.7.4. Regulatory Framework
5.4.7.5. Reimbursement Scenario
5.4.7.6. Thailand T-cell Lymphoma Market, 2021 - 2033
5.5. Latin America
5.5.1. Latin America T-cell Lymphoma Market,, 2021 - 2033
5.5.2. Brazil
5.5.2.1. Key Country Dynamics
5.5.2.2. Target Disease Prevalence
5.5.2.3. Competitive Scenario
5.5.2.4. Regulatory Framework
5.5.2.5. Reimbursement Scenario
5.5.2.6. Brazil T-cell Lymphoma Market, 2021 - 2033
5.5.3. Argentina
5.5.3.1. Key Country Dynamics
5.5.3.2. Target Disease Prevalence
5.5.3.3. Competitive Scenario
5.5.3.4. Regulatory Framework
5.5.3.5. Reimbursement Scenario
5.5.3.6. Argentina T-cell Lymphoma Market, 2021 - 2033
5.6. MEA
5.6.1. MEA T-cell Lymphoma Market,, 2021 - 2033
5.6.2. South Africa
5.6.2.1. Key Country Dynamics
5.6.2.2. Target Disease Prevalence
5.6.2.3. Competitive Scenario
5.6.2.4. Regulatory Framework
5.6.2.5. Reimbursement Scenario
5.6.2.6. South Africa T-cell Lymphoma Market, 2021 - 2033
5.6.3. Saudi Arabia
5.6.3.1. Key Country Dynamics
5.6.3.2. Target Disease Prevalence
5.6.3.3. Competitive Scenario
5.6.3.4. Regulatory Framework
5.6.3.5. Reimbursement Scenario
5.6.3.6. Saudi Arabia T-cell Lymphoma Market, 2021 - 2033
5.6.4. UAE
5.6.4.1. Key Country Dynamics
5.6.4.2. Target Disease Prevalence
5.6.4.3. Competitive Scenario
5.6.4.4. Regulatory Framework
5.6.4.5. Reimbursement Scenario
5.6.4.6. UAE T-cell Lymphoma Market, 2021 - 2033
5.6.5. Kuwait
5.6.5.1. Key Country Dynamics
5.6.5.2. Target Disease Prevalence
5.6.5.3. Competitive Scenario
5.6.5.4. Regulatory Framework
5.6.5.5. Reimbursement Scenario
5.6.5.6. Kuwait T-cell Lymphoma Market, 2021 - 2033
Chapter 6. Competitive Landscape
6.1. Participant’s overview
6.2. Financial performance
6.3. Participant categorization
6.3.1. Market Leaders
6.3.2. T-cell Lymphoma Market Share Analysis, 2023
6.3.3. Company Profiles
6.3.3.1. Acrotech Biopharma
6.3.3.1.1. Company Overview
6.3.3.1.2. Financial Performance
6.3.3.1.3. Product Benchmarking
6.3.3.1.4. Strategic Initiatives
6.3.3.2. Affimed GmbH
6.3.3.2.1. Company Overview
6.3.3.2.2. Financial Performance
6.3.3.2.3. Product Benchmarking
6.3.3.2.4. Strategic Initiatives
6.3.3.3. Bristol Myers Squibb
6.3.3.3.1. Company Overview
6.3.3.3.2. Financial Performance
6.3.3.3.3. Product Benchmarking
6.3.3.3.4. Strategic Initiatives
6.3.3.4. Chipscreen Biosciences
6.3.3.4.1. Company Overview
6.3.3.4.2. Financial Performance
6.3.3.4.3. Product Benchmarking
6.3.3.4.4. Strategic Initiatives
6.3.3.5. Citius Pharma
6.3.3.5.1. Company Overview
6.3.3.5.2. Financial Performance
6.3.3.5.3. Product Benchmarking
6.3.3.5.4. Strategic Initiatives
6.3.3.6. DAIICHI SANKYO COMPANY, LIMITED
6.3.3.6.1. Company Overview
6.3.3.6.2. Financial Performance
6.3.3.6.3. Product Benchmarking
6.3.3.6.4. Strategic Initiatives
6.3.3.7. Eisai Co., Ltd.
6.3.3.7.1. Company Overview
6.3.3.7.2. Financial Performance
6.3.3.7.3. Product Benchmarking
6.3.3.7.4. Strategic Initiatives
6.3.3.8. Genor Biopharma Co. Ltd
6.3.3.8.1. Company Overview
6.3.3.8.2. Financial Performance
6.3.3.8.3. Product Benchmarking
6.3.3.8.4. Strategic Initiatives
6.3.3.9. Innate Pharma
6.3.3.9.1. Company Overview
6.3.3.9.2. Financial Performance
6.3.3.9.3. Product Benchmarking
6.3.3.9.4. Strategic Initiatives
6.3.3.10. Dizal Pharma
6.3.3.10.1. Company Overview
6.3.3.10.2. Financial Performance
6.3.3.10.3. Product Benchmarking
6.3.3.10.4. Strategic Initiatives
6.3.4. Strategy Mapping
6.3.4.1. Expansion
6.3.4.2. Acquisition
6.3.4.3. Collaborations
6.3.4.4. Product/Service Launch
6.3.4.5. Partnerships
6.3.4.6. Others