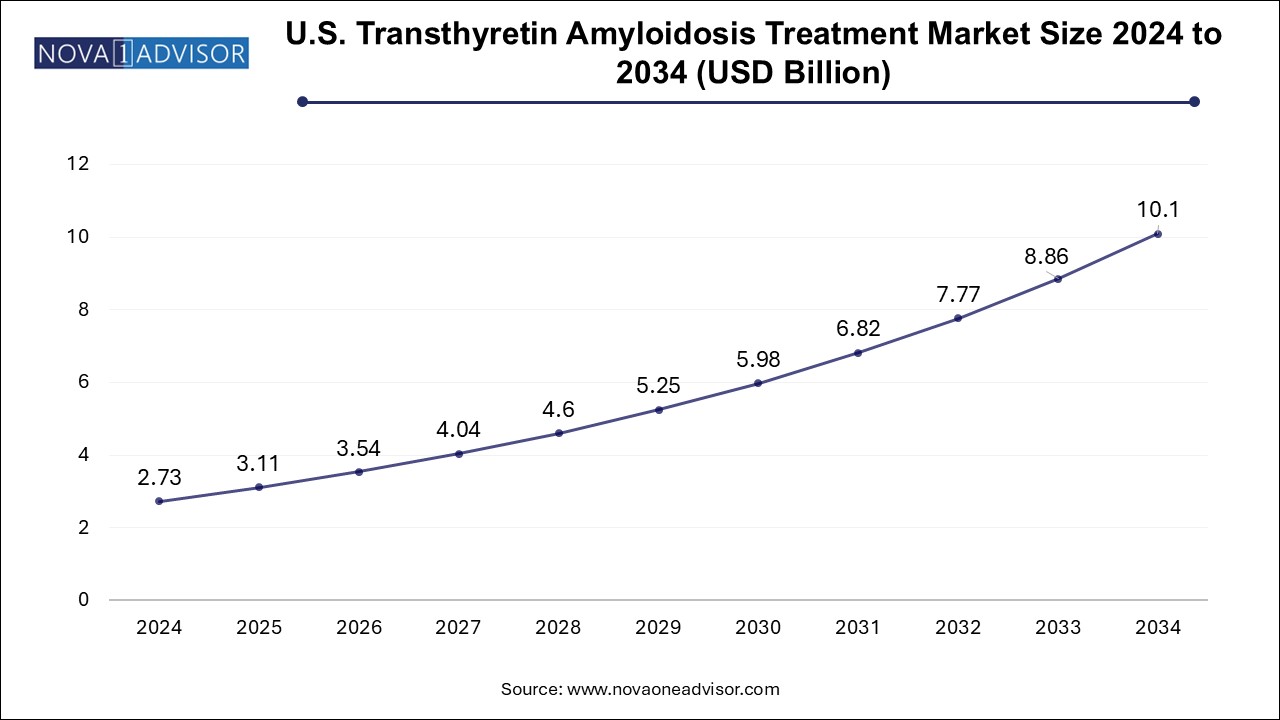
The transthyretin amyloidosis treatment market size was exhibited at USD 7.57 billion in 2024 and is projected to hit around USD 28.06 billion by 2034, growing at a CAGR of 14.0% during the forecast period 2025 to 2034.
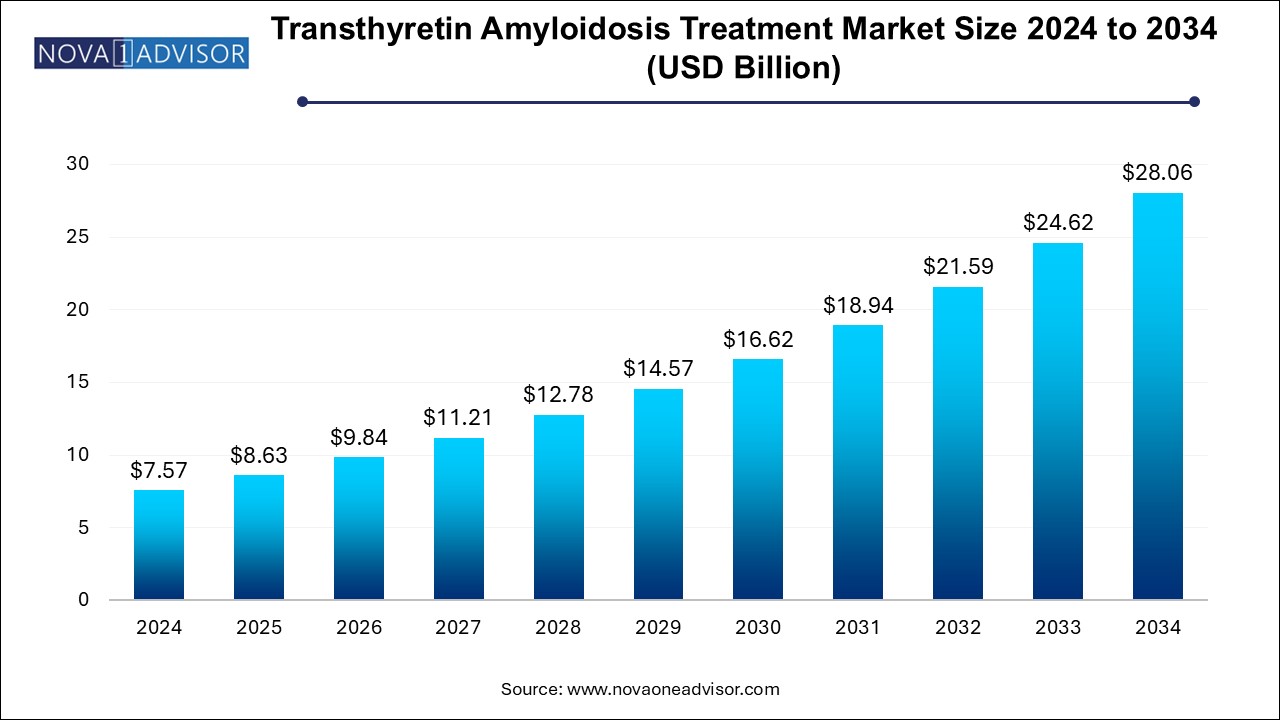
The U.S. transthyretin amyloidosis treatment market size is evaluated at USD 2.73 billion in 2024 and is projected to be worth around USD 10.1 billion by 2034, growing at a CAGR of 12.62% from 2025 to 2034.
North America dominates the transthyretin amyloidosis treatment market, driven by early drug approvals, high healthcare spending, and well-established rare disease infrastructure. The U.S. in particular has been a frontrunner, with the FDA approving tafamidis in 2019, followed by subsequent authorizations of Onpattro, Tegsedi, and Amvuttra. The presence of leading market players, robust clinical trial networks, and active patient advocacy groups like the Amyloidosis Research Consortium have further accelerated treatment uptake.
Strong reimbursement frameworks, including coverage by Medicare and commercial payers for orphan drugs, enhance accessibility in the U.S. Moreover, the region’s focus on value-based care and advanced cardiac imaging capabilities ensures that patients are diagnosed earlier and treated more aggressively, maintaining North America’s leadership in market revenue.
Asia Pacific is the fastest-growing region in the ATTR treatment market, driven by increasing awareness, improved diagnostic capabilities, and expanding patient access programs. Japan has long recognized ATTR due to high prevalence in specific regions, and its universal healthcare coverage supports early treatment adoption. China and India are witnessing rapid diagnostic advancements, especially in urban centers, where genetic testing and cardiac imaging are becoming more accessible.
Global companies are increasingly partnering with local pharmaceutical firms to expand their footprint in Asia. In addition, regional health authorities are adopting faster regulatory pathways for rare diseases, supported by growing patient advocacy and public-private collaborations. As life expectancy increases across the region, the prevalence of wtATTR is expected to climb, creating long-term demand.
The Transthyretin Amyloidosis (ATTR) Treatment Market is rapidly emerging as a vital component of the rare disease therapeutics landscape, fueled by the increasing recognition of the disease, advancements in precision medicine, and growing approval of targeted treatments. ATTR is a progressive and potentially fatal condition caused by the misfolding of transthyretin proteins, which form insoluble amyloid fibrils that deposit in peripheral nerves and cardiac tissues. It is broadly classified into hereditary (hATTR) and wild-type (wtATTR) forms, presenting either as polyneuropathy, cardiomyopathy, or a mixed phenotype.
Historically, the ATTR treatment landscape was largely supportive, with limited disease-modifying options. However, the past decade has witnessed groundbreaking progress with the approval of novel RNA interference (RNAi) therapies, transthyretin stabilizers, and antisense oligonucleotides (ASOs). These advancements are reshaping the treatment paradigm, shifting from symptom management to targeted disease intervention. Drugs like Vyndaqel/Vyndamax (tafamidis), Onpattro (patisiran), and Tegsedi (inotersen) have established the foundation for targeted therapy, while Amvuttra (vutrisiran) and Wainua (eplontersen) represent the next generation of subcutaneous therapies.
The ATTR treatment market is expected to witness substantial expansion through 2034, driven by increasing awareness, improved diagnostic capabilities, a growing aging population (prone to wtATTR), and continuous innovation from biopharmaceutical players. The pipeline remains strong, with several candidates in clinical trials, including gene-silencing and gene-editing technologies that promise to further revolutionize treatment outcomes. Despite challenges related to diagnosis and high treatment costs, the market outlook remains highly optimistic as healthcare systems increasingly prioritize rare disease management.
Rising Adoption of RNAi and ASO-Based Therapies in Polyneuropathy and Cardiomyopathy Subtypes
Increasing Focus on Subcutaneous Administration for Enhanced Patient Convenience (e.g., Amvuttra, Wainua)
Expansion of Disease Awareness Campaigns and Patient Registries in North America and Europe
Pharmaceutical Collaborations to Accelerate Clinical Trials and Global Drug Access
Emerging Use of Genetic Testing and Biomarker-Based Diagnostics for Early Detection
Shift Toward Comprehensive Disease Management Including Cardiac Imaging and Multidisciplinary Care
Increased Regulatory Support for Orphan Drugs, Fast-Track Approvals, and Priority Reviews
Strategic Market Entry into Asia Pacific and Latin America by Leading Drug Makers
Introduction of Pipeline Therapies Exploring Gene Editing (CRISPR/Cas9) for Long-Term Disease Suppression
Growing Utilization of Specialty Pharmacies to Handle Storage, Access, and Reimbursement Challenges
| Report Coverage | Details |
| Market Size in 2025 | USD 8.63 Billion |
| Market Size by 2034 | USD 28.06 Billion |
| Growth Rate From 2025 to 2034 | CAGR of 14.0% |
| Base Year | 2024 |
| Forecast Period | 2025-2034 |
| Segments Covered | Therapy, Type, Disease, Distribution Channel, Region |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope | North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled | Pfizer Inc.; Alnylam Pharmaceuticals, Inc.; Ionis Pharmaceuticals; AstraZeneca; Akcea Therapeutics; BridgeBio Inc.; Intellia Therapeutics, Inc.; SOM BIOTECH; Oncopeptides AB; Takeda Pharmaceutical Company Limited |
One of the major drivers for the ATTR treatment market is the rising prevalence of transthyretin amyloidosis, particularly among aging populations, and the associated improvements in diagnostic technologies. While the disease was once considered underdiagnosed and often misclassified as other forms of cardiomyopathy or neuropathy, clinical awareness has grown significantly. Studies now estimate that wild-type ATTR (primarily affecting older men) may be present in up to 10-15% of elderly heart failure patients with preserved ejection fraction (HFpEF), a figure much higher than previously believed.
Advanced diagnostic tools such as technetium-labeled bone scintigraphy, echocardiography with strain imaging, and genetic sequencing are now widely used to differentiate ATTR from other amyloidoses and cardiomyopathies. These techniques, along with heightened clinical suspicion, have enabled early diagnosis, allowing physicians to initiate targeted therapies during the window of reversibility. As diagnostic precision improves across hospitals and specialty centers, the pool of treatable patients expands—fueling market demand for effective therapies.
Despite therapeutic breakthroughs, a key restraint for the ATTR treatment market is the high cost of targeted therapies, which poses a challenge to access and reimbursement, especially in low- and middle-income countries. Medications like tafamidis (Vyndaqel/Vyndamax) are priced at over $200,000 annually in the U.S., making them among the most expensive orphan drugs on the market. RNAi-based drugs like Onpattro and Amvuttra, though clinically effective, also fall into the premium pricing category.
These costs limit accessibility for uninsured or underinsured patients and place a substantial burden on public and private payers. Moreover, coverage disparities across global healthcare systems hinder widespread adoption. Even in high-income regions, budgetary constraints and value-assessment frameworks like those used by the National Institute for Health and Care Excellence (NICE) in the UK can delay patient access to novel therapies. Thus, the economic barrier remains a substantial hurdle for market expansion, particularly in emerging economies.
A significant opportunity lies in the expanding therapeutic pipeline and market entry into underpenetrated regions, such as Asia Pacific, Latin America, and Eastern Europe. Biopharma companies are actively developing next-generation compounds with improved safety profiles, less frequent dosing, and broader applicability across both hATTR and wtATTR populations. Several candidates in Phase II/III trials promise once-quarterly dosing, gene-silencing capabilities, or dual cardiomyopathy-polyneuropathy efficacy.
Moreover, leading players have an opportunity to secure first-mover advantage in countries where regulatory frameworks are evolving, and awareness is still nascent. Companies that invest early in physician training, diagnostic support, and patient advocacy partnerships can establish strong brand recognition and loyalty. In markets like India, China, and Brazil—where ATTR cases are likely underreported—there is significant untapped potential. Launch strategies that include compassionate use programs, local manufacturing, and tiered pricing models could drive early adoption and market share growth.
Targeted therapy currently dominates the transthyretin amyloidosis treatment market, accounting for the largest revenue share. Within this category, Vyndaqel/Vyndamax (tafamidis) leads due to its broad indication for ATTR-CM and strong clinical data demonstrating reduced mortality and cardiovascular hospitalization. Approved by the FDA and EMA, tafamidis has become a first-line treatment for cardiomyopathy patients. Similarly, Onpattro (patisiran) and Amvuttra (vutrisiran), both RNAi-based therapies from Alnylam Pharmaceuticals, are widely used for ATTR-PN. Their ability to reduce serum TTR levels by over 80% has revolutionized polyneuropathy treatment.
Meanwhile, pipeline therapies are expected to be the fastest-growing segment as emerging candidates enter late-stage trials. These include antisense oligonucleotides such as eplontersen (Wainua) developed by Ionis and AstraZeneca, and gene-editing candidates exploring CRISPR-based technologies. The pipeline’s strength lies in its focus on improving administration routes, safety, and dosing convenience—factors that will likely drive future patient preference and market capture.
Transthyretin Amyloidosis Treatment Market By Type Insights
ATTR with Cardiomyopathy (ATTR-CM) is the dominant type segment, driven by the high prevalence of wtATTR among the aging population. Cardiac amyloidosis presents with symptoms mimicking heart failure and has long been underdiagnosed. With improved detection via imaging and biopsy, more patients are receiving targeted treatments like tafamidis. Given that ATTR-CM contributes to functional decline, hospitalizations, and mortality, the therapeutic focus and commercial attention remain high.
However, ATTR with Polyneuropathy (ATTR-PN) is experiencing the fastest growth. Treatments like Onpattro, Amvuttra, and Tegsedi have been instrumental in slowing disease progression, improving quality of life, and delaying disability. Patients with hATTR-PN typically present earlier and respond well to RNAi-based therapy, which has seen rapid uptake, particularly in North America and Europe. Ongoing trials evaluating combination therapies may further enhance outcomes, driving continued momentum in this segment.
Wild type amyloidosis held the largest market share of 55.2% in 2024, especially in the cardiomyopathy context. It is primarily a disease of elderly men and is gaining clinical recognition due to its link to unexplained heart failure with preserved ejection fraction (HFpEF). As diagnostic tools improve and the geriatric population expands, wtATTR is expected to see rising treatment demand, further supported by recent label expansions for drugs like tafamidis and vutrisiran.
Hereditary transthyretin amyloidosis is expected to register the fastest CAGR of 13.9% over the forecast period, owing to the earlier availability of therapies and the relative ease of genetic diagnosis. This form affects both peripheral nerves and the heart, often presenting in younger adults, especially in high-incidence regions such as Portugal, Sweden, and Japan. With family screening and robust patient registries, early diagnosis and treatment initiation are common in hATTR cases.
Hospital pharmacies dominated the market and accounted for a share of 51.0% in 2024, requirement for administration in controlled environments (particularly for injectable and RNAi therapies), and ongoing patient monitoring. Most therapies are initiated in tertiary care settings or specialty amyloidosis centers, reinforcing the dominance of hospital distribution.
Online pharmacies are projected to grow at the fastest CAGR of 14.8% over the forecast period. As treatments shift toward subcutaneous formulations like Amvuttra and Wainua, which can be administered at home, the convenience of home delivery through accredited online platforms is gaining traction. Specialty pharmacy models with integrated patient education, logistics support, and reimbursement handling are accelerating this trend.
March 2025: Alnylam Pharmaceuticals announced the expansion of Amvuttra's label to include ATTR-CM in addition to ATTR-PN, following positive Phase III results demonstrating cardiac function preservation.
February 2025: Ionis Pharmaceuticals and AstraZeneca received FDA approval for Wainua (eplontersen), a subcutaneous ASO therapy for ATTR-PN, marking a significant addition to the competitive pipeline.
January 2025: Pfizer initiated a global Phase IV registry to assess real-world outcomes for Vyndamax in patients with wild-type ATTR-CM across 12 countries.
December 2024: BridgeBio Pharma reported encouraging data from its Phase II trial of a next-gen TTR stabilizer for both hereditary and wild-type forms, with plans to file for accelerated approval in 2026.
November 2024: Silence Therapeutics entered Phase I trials for its siRNA-based therapy targeting TTR production, aiming for quarterly dosing as a next-gen alternative to Amvuttra.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the transthyretin amyloidosis treatment market
By Therapy
By Type
By Disease
By Distribution Channel
By Regional