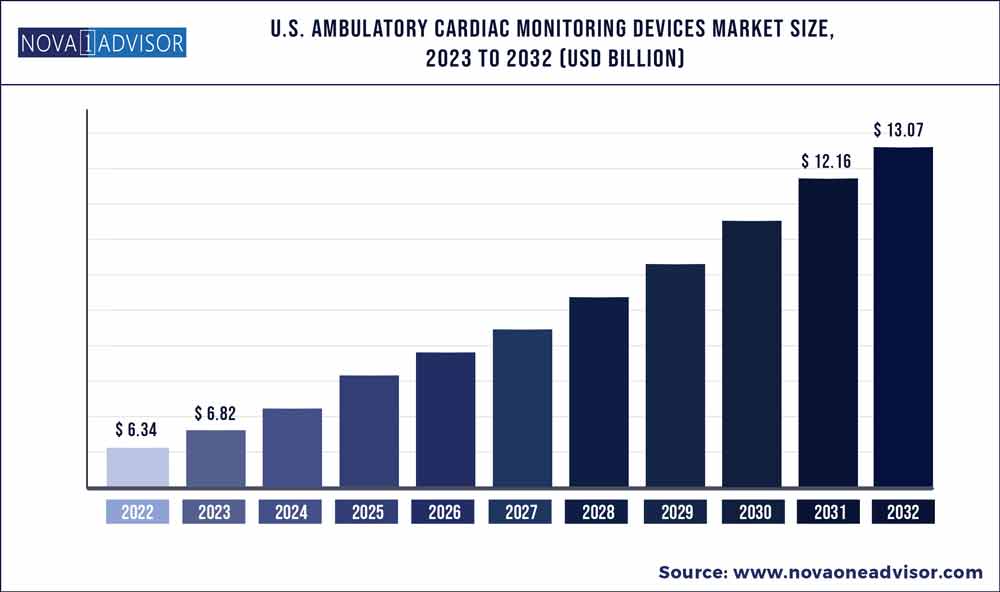
The U.S. ambulatory cardiac monitoring devices market size was estimated at USD 6.34 billion in 2022 and is expected to surpass around USD 13.07 billion by 2032 and poised to grow at a compound annual growth rate (CAGR) of 7.5% during the forecast period 2023 to 2032.
Ambulatory cardiac monitor is an electrocardiogram that records the rhythm of the heart. The length of recording time and the ability to send recordings over the phone are two features that set it apart. Ambulatory cardiac monitors are used by most professional experts to assess heartbeat over time, correlate signs with the heartbeat, make a diagnosis of abnormal heart rhythms, and evaluate other symptoms related to the heart. These devices help monitor the heart with increased detection time and more precise results as compared to traditional heart monitors.
Ambulatory monitoring devices allow for a new healthcare paradigm by gathering and examining lengthy data for trustable diagnostics. These devices are gaining popularity for continuous cardiac disease monitoring. Recent advancements have enabled solutions that are both affordable and dependable, enabling vulnerable populations to be monitored from the comfort of their own homes. These devices detect important physiological events early, resulting in timely alerts to seek medical attention.
U.S. Ambulatory Cardiac Monitoring Devices Market Report Scope
|
Report Coverage |
Details |
|
Market Size in 2023 |
USD 6.82 Billion |
|
Market Size by 2032 |
USD 13.07 Billion |
|
Growth Rate From 2023 to 2032 |
CAGR of 7.5% |
|
Base Year |
2022 |
|
Forecast Period |
2023 to 2032 |
|
Segments Covered |
Product, Application, End-user |
|
Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
|
Report Coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Key Companies Profiled |
Abbott Laboratories, Biotronik, Boston Scientific Corporation, GE Healthcare, Hill-Rom Holdings, Koninklijke Philips N.V., Medtronic,Nihon Kohden Corporation |
Increase in Demand for Long-term ECG Monitoring and Self-care Management Driving U.S. Market
An electrocardiography (ECG) device uses electrical impulses to record heartbeats, which are then amplified and displayed on an ECG display. ECG devices are used to detect various heart diseases and arrhythmias in a person's body and to help choose the best treatment. Electrocardiograms can presently obtained at home using a portable ECG device, thanks to advances in technology. According to the World Health Organization, cardiovascular diseases affect more than 60% of the global population.
Moreover, Mobile Cardiac Telemetry (MCT) is a constant form of ambulatory care ECG monitoring that can last a maximum of 30 days. MCT is a relatively new form of ambulatory cardiac monitoring that has been demonstrated to be a reliable and efficient way to monitor a patient's ECG data while the patient is at work or sleeping.
Various smart portable EKG monitoring devices are available, such as EMAY Portable EKG Monitoring Device (for iPhone & Android, Mac & Windows. This portable ECG monitor is small enough to fit in a person's pocket. It is compatible with majority of smartphones. It also has the ability to save, view, and share reports, and record 30 seconds of a person's heart rate and rhythm. This device is activated by resting one's hands on the sensors on either side of the ECG monitor for 30 seconds. The information can then be transferred to the smartphone. These factors are likely to propel ambulatory cardiac monitoring devices market demand in the U.S.
Measures to Reduce Infections Due to Cardiac Implantable Electronic Devices Fueling U.S. Market
Cardiac implantable electronic devices (CIEDs) have been linked to increased survival and quality of life when used to treat cardiac arrhythmias. However, CIED-related infection is a significant concern. Infections from CIEDs are most common in patients who have a cardiac resynchronization therapy defibrillator or an implantable cardioverter defibrillator. According to the European Heart Journal (CRTDs), the rate of CIED infections in pacemakers was 1.19% in implantable cardioverter-defibrillators, 2.18% in cardiac resynchronization therapy pacemakers (CRT-Ps), and 3.35% in CRT defibrillators.
Medtronic provides an absorbable, multifilament mesh envelope to improve CIED stabilization in the subcutaneous pocket and to elute the antibiotics minocycline and rifampin (TYRX Absorbable Antibacterial Envelope). These envelopes are designed to be used in conjunction with cardiac implantable electronic devices (CIEDs), such as pacemakers and implantable cardioverter defibrillators (ICDs). In 2019, an international randomized controlled trial discovered that the envelope reduced the incidence of infection by 40% when compared to standard-of-care infection prevention alone. These factors are propelling the ambulatory cardiac monitoring devices market share in the U.S.
Technological Advancements Bolstering Mobile Cardiac Telemetry Segment
In terms of product, the ambulatory cardiac monitoring devices market has been classified into ECG devices, Holter monitors, event monitors, implantable loop recorders, and mobile cardiac telemetry. Mobile cardiac telemetry is a relatively new technology in the field of ambulatory cardiac monitoring. This technology is significant because it is a real-time ECG monitor, capable of automatically detecting any ECG abnormalities and automatically transmitting this ECG information via cell phone technology to an accredited diagnostic laboratory for professional review.
Mobile cardiac telemetry monitoring is the method of the future because it has several technological advantages over other long-term monitoring options. MCT monitoring technology captures cardiac abnormalities in real-time, making it a superior method for detecting ECG abnormalities. The accelerated nature of MCT could reduce long-term healthcare costs and improve diagnostic care. This in turn is likely to augment the wireless ambulatory telemetry monitors market.
Increase in Demand for Ambulatory Diagnosis and Management of Cardiac Arrhythmias Driving Arrhythmia Segment
Based on application, the ambulatory cardiac monitoring devices market in the U.S. has been segregated into arrhythmia, coronary artery disease, hypertension, and others. Electrocardiographic monitoring devices can be utilized effectively for management of cardiac arrhythmias and ambulatory diagnosis. Palpitations, hypotension, antiarrhythmic drug monitoring, and arrhythmia surveillance in patients with suspected arrhythmias are all potential indications.
Ambulatory arrhythmia monitoring devices track heartbeats in hospitals, ASCs, homecare, and other settings. The ambulatory cardiac monitoring devices market trends in the U.S. indicate that the demand for these devices is expected to be driven by increase in the geriatric population and rise in the prevalence of cardiovascular disorders. Therefore, the demand for effective monitoring systems is expected to increase in the near future. Furthermore, the development of efficient medical devices is aided by the improvement of healthcare infrastructure, which could promote industry growth in the near future.
Rise in Number of Patients Seeking Hospital Care Propelling Hospitals Segment
In terms of end-user, the ambulatory cardiac monitoring devices market in the U.S. has been divided into hospitals, clinics, ambulatory surgery centers, and others. Some of the ambulatory cardiac monitoring devices used in hospitals are electrocardiograms, blood pressure monitors, electronic fetal monitors, pulse oximeters, wearables, body temperature monitors, and others. Ambulatory cardiac monitoring devices are one of the most important medical devices, saving millions of lives every day. Every hospital requires a monitoring system in order to provide the best possible care to their patients.
Analysis of Key Players
The ambulatory cardiac monitoring devices market in the U.S. is consolidated, with the presence of a small number of leading players. Most of the companies are investing significantly in R&D activities, primarily to introduce advanced ambulatory cardiac monitoring devices in the country. Key players are engaging in strategic alliances to increase revenue and market share.
Furthermore, diversification of product portfolios and mergers & acquisitions are prominent strategies adopted by key players. Abbott Laboratories, Biotronik, Boston Scientific Corporation, GE Healthcare, Hill-Rom Holdings, Koninklijke Philips N.V., Medtronic, and Nihon Kohden Corporation are prominent players operating in the U.S. market
Key Developments
In June 2022, GE Healthcare announced the launch of Portrait Mobile, a wireless patient monitoring system that enables continuous monitoring throughout a patient’s stay. The system helps clinicians detect patient deterioration. Early detection of patient deterioration could help reduce length of stay and intensive care unit (ICU) admissions and improve patient outcomes.
In July 2021, Abbott Laboratories announced the launch of its latest implantable cardiac monitor (ICM), the Jot Dx, in the U.S. Doctors and hospitals can use Jot Dx ICM to view all abnormal cardiac rhythm data or use the ‘Key Episodes’ option to inform via a unique feature that simplifies recorded arrhythmia rhythms. This technology allows for remote detection of cardiac arrhythmias in patients as well as improved diagnostic accuracy. SyncUP, a personalized service that provides customized education and training to help patients connect to and stay connected to the ICM, is used by Jot Dx ICM.
Some of the prominent players in the U.S. Ambulatory Cardiac Monitoring Devices Market include:
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the U.S. Ambulatory Cardiac Monitoring Devices market.
By Product
By Application
By End-user