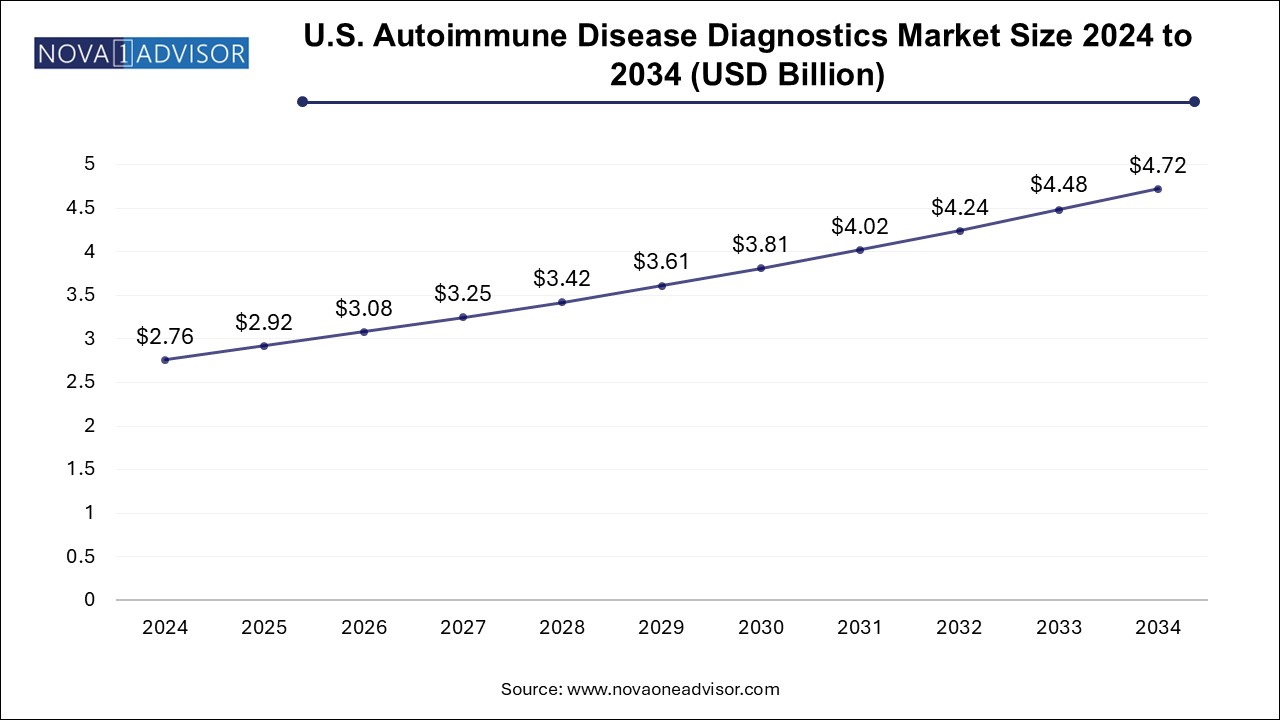
The U.S. autoimmune disease diagnostics market size was exhibited at USD 2.76 billion in 2024 and is projected to hit around USD 4.72 billion by 2034, growing at a CAGR of 5.4% during the forecast period 2025 to 2034.
| Report Coverage | Details |
| Market Size in 2025 | USD 2.92 Billion |
| Market Size by 2034 | USD 4.72 Billion |
| Growth Rate From 2025 to 2034 | CAGR of 5.4% |
| Base Year | 2024 |
| Forecast Period | 2025-2034 |
| Segments Covered | Type, Test Type, End-use, Region |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Key Companies Profiled | Abbott Laboratories; Biomerieux; Trinity Biotech; Bio-rad Laboratories; Thermo Fisher Scientific; BD Biosciences; Beckman Coulter; F. Hoffmann-La Roche Ltd.; Quest Diagnostics Inc.; Siemens Healthcare GmbH; Werfen |
The rising prevalence & awareness of autoimmune diseases, increasing emphasis on early diagnosis, and a shift towards personalized & precision medicine are driving the market growth worldwide. According to the study published by the University of Oxford, in May 2023, autoimmune diseases affect 1 in 10 people among the total population of 22 million people. Autoimmune diseases including type 1 diabetes have registered to occur more frequently over the recent years.
According to the Atlas of Multiple Sclerosis Report, nearly 1.0 million in the U.S. are currently living with multiple sclerosis. Autoimmune diseases are becoming more prevailing, which is a challenge for the country. One of the main factors driving use rates is the increased understanding of autoimmune diseases as an outcome of programs for education, research, activism, and support.
The development and introduction of advanced diagnostic technologies, such as molecular diagnostics, next-generation sequencing, and immunodiagnostics, have significantly improved the accuracy and efficiency of autoimmune disease detection. These advancements have fueled the growth of the market. For instance, in October 2023, WellTheory, a platform dedicated to alleviating symptoms of autoimmune diseases introduced a new enterprise solution tailored for employers and payers seeking to provide autoimmune care. The newly launched product is designed to address the hidden expenses and productivity repercussions associated with untreated autoimmune conditions. It will encompass a community-centered initiative that grants members access to various resources such as events, tools, experts, and peer support.
Furthermore, the increasing concerns over autoimmune conditions in the country, together with growing alertness about diseases and health generates the requirement for diagnostics for prompt disease detection and therefore propels the market growth. The American Autoimmune Related Disease Association (AARDA), a prominent organization dedicated to the elimination of autoimmune diseases and the alleviation of pain associated with these conditions, designates the month of March as the Autoimmune Disease Awareness Month (ADAM). This initiative aims to raise public awareness, support those affected by autoimmune diseases, and encourage research and advancements in treatment options in the country. In February 2021, the American Kidney Fund (AKF) introduced a patient-focused awareness and education movement on lupus nephritis, a kidney illness.
The market in the U.S. is expected to grow substantially over the forecast period due to the growing focus on the effective treatment options for autoimmune diseases. According to the Penn Medicine News published in August 2023, Penn Medicine has shifted its focus to autoimmunity, which includes over 80 illnesses that impact over 20 million Americans. By focusing on the underlying causes of patients' illnesses, cutting-edge researchers like Payne hope to give patients less harmful, possibly longer-lasting, or even permanent treatment.
The market is poised for growth in the forecast period, despite challenges such as slow result turnaround times. However, the necessity for multiple diagnostic tests, high costs, reimbursement issues, and regulatory uncertainties are expected to impede its progress.
Localized autoimmune disease diagnostics dominated the market and accounted for a share of 66.15% in 2024. This high share can be attributed to the rising adoption of advanced imaging techniques, such as ultrasound, MRI, and PET scans, for detailed visualization of affected tissues or organs. The development of molecular diagnostic tools, such as polymerase chain reaction (PCR) and next-generation sequencing (NGS), enables the identification of specific autoantibodies or genetic markers associated with localized autoimmune diseases. In the medical field, there are various specialists who concentrate on particular organs or systems of the body. These specialists include endocrinologists, gastroenterologists, neurologists, and rheumatologists, among others. They are responsible for diagnosing, managing, and treating diseases related to their specific organ or system.
The systemic autoimmune disease diagnostics is expected to grow at the fastest CAGR over the forecast period. The growth is attributed to the growing advancements in technology, rising demand for systemic autoimmune disease diagnostics, and supportive government policies. The systemic autoimmune disease diagnostics is further segmented into rheumatoid arthritis, ankylosing spondylitis diagnostics, systemic lupus erythematosus (SLE), and others.
In an extremely competitive landscape, a wide array of players, ranging from established industry giants to innovative startups, compete for market dominance. This intense competition fosters a continuous drive for innovation and excellence as companies aim to position themselves apart through maximum product quality, strategic pricing approaches, and exceptional customer satisfaction. In September 2021, Scipher Medicine entered into a partnership with Ventegra Inc. with the aim of benefiting Ventegra’s customers by providing them access to Scipher’s PrismRA liquid molecular signature test. This test plays a crucial role in determining the most effective treatment options for patients suffering from rheumatoid arthritis (RA). As a result of this strategic alliance, the market growth for personalized medicine and rheumatoid arthritis treatments is expected to grow substantially.
Antinuclear antibody tests accounted for the largest market revenue share in 2024 and are expected to grow at the fastest CAGR over the forecast period. This is attributable to the growing government initiatives and the increasing incidences of autoimmune diseases. As these disorders become more prevalent, the demand for ANA testing grows, as it is a crucial diagnostic tool for identifying and monitoring these conditions. For instance, as per a CDC study, approximately 3,000 to 6,000 individuals in the U.S. are diagnosed with Guillain-Barre syndrome (GBS) each year.
Furthermore, according to a study published by the National Institute of Health (NIH) in April 2020, the most prevalent indicator of autoimmunity, antinuclear antibodies (ANA), was becoming much more widespread in the U.S. overall and in some specific populations. The study examined how the presence of ANA changes over time in a representative sample of Americans, including males, non-Hispanic Whites, adults aged 50 and above, and teenagers.
The hospitals segment dominated the market share of 55.0% in 2024. This is attributable to the growingneed for accurate and precise testing methods. Autoimmune diseases often present with nonspecific symptoms, making it crucial to rely on specific diagnostic tests to confirm the presence of these conditions. Hospitals prioritize the use of reliable laboratory tests, such as autoantibody assays and immunological markers, to accurately diagnose autoimmune diseases and differentiate them from other disorders.
Diagnostic centers are projected to grow at the fastest CAGR over the forecast period. The growing demand for easyaccessibility and convenience is driving the segment growth.Centers that offer convenient appointment scheduling, shorter wait times for test results, and easy access to follow-up consultations with specialists are often favored by patients. Diagnostic centers offer a wide range of specialized tests for autoimmune diseases and are often preferred by end users. These tests include autoantibody testing, inflammation markers, genetic testing, and specific antigen tests that are essential for diagnosing different autoimmune conditions accurately further driving the market growth.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the U.S. autoimmune disease diagnostics market
Type
Test Type
End-use
Regional
Chapter 1. Methodology and Scope
1.1. Market Segmentation & Scope
1.2. Segment Definitions
1.2.1. Type
1.2.2. Test Type
1.2.3. End-use
1.2.4. Estimates and forecasts timeline
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased database
1.4.2. internal database
1.4.3. Secondary sources
1.4.4. Primary research
1.5. Information or Data Analysis
1.5.1. Data analysis models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.2. Volume price analysis (Model 2)
1.8. List of Secondary Sources
1.9. List of Primary Sources
1.10. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.2.1. Test outlook
2.2.2. Test type outlook
2.2.3. End-use outlook
2.3. Competitive Insights
Chapter 3. U.S. Autoimmune Disease Diagnostics Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook
3.2. Market Dynamics
3.2.1. Market driver analysis
3.2.1.1. Presence of research funding programs
3.2.1.2. Presence of favorable government initiatives
3.2.1.3. Growing patient awareness levels
3.2.1.4. Technological innovation in the form of lab automation
3.2.2. Market restraint analysis
3.2.2.1. Slow diagnostic result turnaround times and lack of skilled
3.3. U.S. Autoimmune Disease Diagnostics Market Analysis Tools
3.3.1. Industry Analysis - Porter’s Five Forces
3.3.1.1. Supplier power
3.3.1.2. Buyer power
3.3.1.3. Substitution threat
3.3.1.4. Threat of new entrant
3.3.1.5. Competitive rivalry
3.3.2. PESTEL Analysis
3.3.2.1. Political landscape
3.3.2.2. Technological landscape
3.3.2.3. Economic landscape
Chapter 4. U.S. Autoimmune Disease Diagnostics Market: Type Estimates & Trend Analysis
4.1. Type Market Share, 2024 & 2034
4.2. Segment Dashboard
4.3. U.S. Autoimmune Disease Diagnostics Market by Type Outlook
4.4. Market Size & Forecasts and Trend Analyses, 2021 to 2034 for the Following
4.4.1. U.S. Autoimmune Disease Diagnostics
4.4.1.1. Market estimates and forecasts 2021 to 2034
4.4.1.2. Rheumatoid arthritis
4.4.1.2.1. Market estimates and forecasts 2021 to 2034
4.4.1.3. Ankylosing spondylitis diagnostics
4.4.1.3.1. Market estimates and forecasts 2021 to 2034
4.4.1.4. Systemic lupus erythematosus (SLE)
4.4.1.4.1. Market estimates and forecasts 2021 to 2034
4.4.1.5. Others
4.4.1.5.1. Market estimates and forecasts 2021 to 2034
4.4.2. Localized autoimmune disease diagnostics
4.4.2.1. Market estimates and forecasts 2021 to 2034
4.4.2.2. Multiple sclerosis
4.4.2.2.1. Market estimates and forecasts 2021 to 2034
4.4.2.3. Type 1 diabetes
4.4.2.3.1. Market estimates and forecasts 2021 to 2034
4.4.2.4. Hashimoto's Thyroiditis
4.4.2.4.1. Market estimates and forecasts 2021 to 2034
4.4.2.5. Idiopathic thrombocytopenic purpura
4.4.2.5.1. Market estimates and forecasts 2021 to 2034
4.4.2.6. Others
4.4.2.6.1. Market estimates and forecasts 2021 to 2034
Chapter 5. U.S. Autoimmune Disease Diagnostics Market: Test Type Estimates & Trend Analysis
5.1. Test Type Market Share, 2024 & 2034
5.2. Segment Dashboard
5.3. U.S. Autoimmune Disease Diagnostics Market by Test Type Outlook
5.4. Market Size & Forecasts and Trend Analyses, 2021 to 2034 for the Following
5.4.1. Antinuclear antibody tests
5.4.1.1. Market estimates and forecasts 2021 to 2034
5.4.2. Autoantibody tests
5.4.2.1. Market estimates and forecasts 2021 to 2034
5.4.3. C-reactive Protein (CRP)
5.4.3.1. Market estimates and forecasts 2021 to 2034
5.4.4. Complete blood count (CBC)
5.4.4.1. Market estimates and forecasts 2021 to 2034
5.4.5. Urinalysis
5.4.5.1. Market estimates and forecasts 2021 to 2034
5.4.6. Others
5.4.6.1. Market estimates and forecasts 2021 to 2034
Chapter 6. U.S. Autoimmune Disease Diagnostics Market: End-use Estimates & Trend Analysis
6.1. End-use Market Share, 2024 & 2034
6.2. Segment Dashboard
6.3. U.S. Autoimmune Disease Diagnostics Market by End-use Outlook
6.4. Market Size & Forecasts and Trend Analyses, 2021 to 2034 for the Following
6.4.1. Hospitals
6.4.1.1. Market estimates and forecasts 2021 to 2034
6.4.2. Diagnostic centers
6.4.2.1. Market estimates and forecasts 2021 to 2034
6.4.3. Others
6.4.3.1. Market estimates and forecasts 2021 to 2034
Chapter 7. U.S. Autoimmune Disease Diagnostics Market: Region Estimates & Trend Analysis
7.1. Regional Market Share, 2024 & 2034
7.2. U.S. Autoimmune Disease Diagnostics Market by Region Outlook
7.3. Market Size & Forecasts and Trend Analyses, 2021 to 2034 for the Following
7.3.1. West
7.3.1.1. Market estimates and forecasts 2021 to 2034
7.3.2. Midwest
7.3.2.1. Market estimates and forecasts 2021 to 2034
7.3.3. Northeast
7.3.3.1. Market estimates and forecasts 2021 to 2034
7.3.4. Southwest
7.3.4.1. Market estimates and forecasts 2021 to 2034
7.3.5. Southeast
7.3.5.1. Market estimates and forecasts 2021 to 2034
Chapter 8. Competitive Landscape
8.1. Recent Developments & Impact Analysis, By Key Market Participants
8.2. Company/Competition Categorization
8.3. Vendor Landscape
8.3.1. List of key distributors and channel partners
8.3.2. Key customers
8.3.3. Key company heat map analysis, 2024
8.4. Company Profiles
8.4.1. Medtronic
8.4.1.1. Company overview
8.4.1.2. Financial performance
8.4.1.3. Product benchmarking
8.4.1.4. Strategic initiatives
8.4.2. Boston Scientific Corporation
8.4.2.1. Company overview
8.4.2.2. Financial performance
8.4.2.3. Product benchmarking
8.4.2.4. Strategic initiatives
8.4.3. Johnson & Johnson Service Inc. (Ethicon, Inc.)
8.4.3.1. Company overview
8.4.3.2. Financial performance
8.4.3.3. Product benchmarking
8.4.3.4. Strategic initiatives
8.4.4. AngioDynamics
8.4.4.1. Company overview
8.4.4.2. Financial performance
8.4.4.3. Product benchmarking
8.4.4.4. Strategic initiatives
8.4.5. Bioventus Inc. (Misonix Inc.)
8.4.5.1. Company overview
8.4.5.2. Financial performance
8.4.5.3. Product benchmarking
8.4.5.4. Strategic initiatives
8.4.6. EDAP TMS
8.4.6.1. Company overview
8.4.6.2. Financial performance
8.4.6.3. Product benchmarking
8.4.6.4. Strategic initiatives
8.4.7. Chongqing Haifu Medical Technology Co., Ltd
8.4.7.1. Company overview
8.4.7.2. Financial performance
8.4.7.3. Product benchmarking
8.4.7.4. Strategic initiatives
8.4.8. Mermaid Medical
8.4.8.1. Company overview
8.4.8.2. Financial performance
8.4.8.3. Product benchmarking
8.4.8.4. Strategic initiatives
8.4.9. HealthTronics, Inc.
8.4.9.1. Company overview
8.4.9.2. Financial performance
8.4.9.3. Product benchmarking
8.4.9.4. Strategic initiatives
8.4.10. H.S. Hospital Service S.p.A.
8.4.10.1. Company overview
8.4.10.2. Financial performance
8.4.10.3. Product benchmarking
8.4.10.4. Strategic initiatives